Influenza virosome coated bionic nano vaccine and preparation method thereof
A kind of influenza virus, bionic nano technology, applied in biochemical equipment and methods, virus/bacteriophage, vaccine, etc., can solve the problem of low pDNA expression efficiency, and achieve the effect suitable for large-scale production, low production cost and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
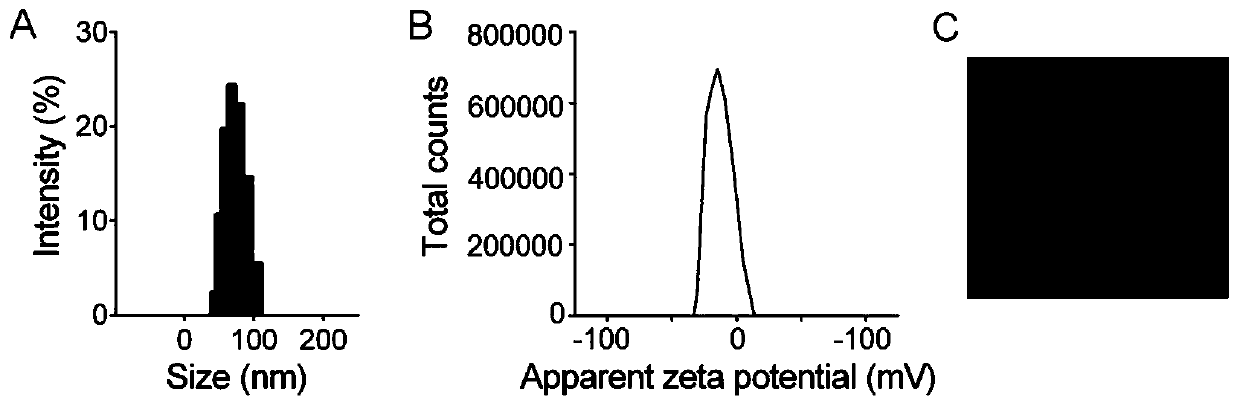
[0059] (1) Preparation of VI: Influenza virus PR8 was ultracentrifuged, and the bottom precipitate was collected. For every 5 mg of precipitated virus, 375 μL of 200 mM 1,2-dihexanoylphosphatidylcholine was added for 30 minutes on ice. Collect the supernatant by ultracentrifugation, dialyze the supernatant in HBS buffer for 24 hours, remove 1,2-dicaproyl lecithin, and obtain recombinant VI, wherein the ultracentrifugation conditions are 4°C, 100,000×g, 1.5h;
[0060] (2) Synthesis of fluorinated cationic polymer: react according to the molar ratio of amino group, heptafluorobutyric anhydride and triethylamine in the cationic polymer is 1:3:1.2, the reaction medium is methanol, and stirred at room temperature for 48 hours. After the reaction, the reaction product was dialyzed in deionized water with a pH of 3 to 4 for 2 days, and freeze-dried to obtain a fluorinated modified cationic polymer. The cationic polymer used in this example was 25kDa PEI;
[0061] (3) FAu preparation:...
Embodiment 2
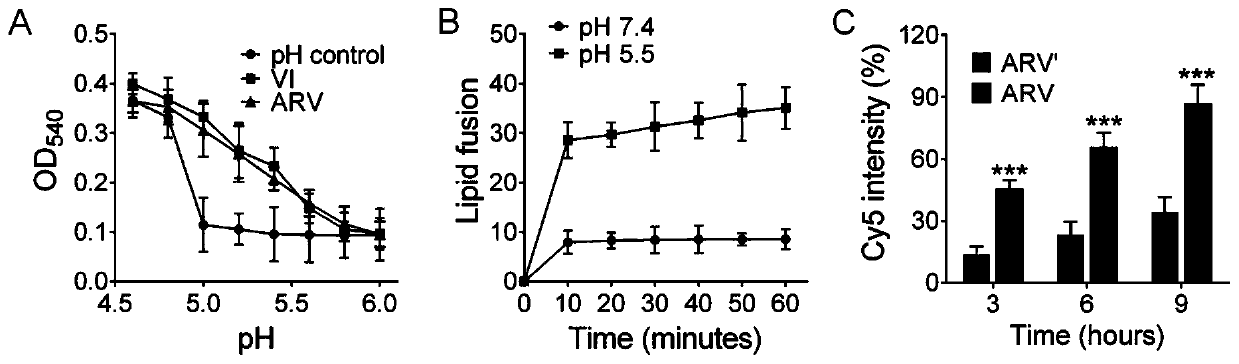
[0065] Low pH conditions in cellular endosomes / lysosomes can induce conformational changes in influenza virus hemagglutinin (HA), causing fusion of the viral envelope with endosome / lysosome membranes. Therefore, under acidic conditions, when the conformation of HA adsorbed on red blood cells changes, it will cause the red blood cell membrane to rupture, thereby releasing hemoglobin and causing hemolysis. Thus, the fusion process of the membrane can be simulated by using the hemolysis experiment. Such as image 3 As shown in A, ARV and VI prepared in Example 1 were incubated with red blood cells respectively, OD 540 UV absorption increased with the decrease of pH, both ARV and Ⅵ had hemolytic effect, which indicated that membrane fusion had occurred. Further, anionic liposomes are used to simulate the membrane structure of cells and carry fluorescence resonance energy transfer (FRET) compounds, which are composed of DOPC, cholesterol and FRET reagent pair NBD-PE and Rho-PE, w...
Embodiment 3
[0067] The ARV prepared in Example 1 was immunized into mice (C57BL / 6, 20±2 g, male) by subcutaneous injection at the base of the tail at the first week and the third week, and each dose was 40 μg of ARV protein. Isolate the spleen at the 4th week, prepare a single cell suspension, collect 2 million cells into a 1.5mL centrifuge tube, centrifuge at 1600rpm for 5min, remove the supernatant, wash gently with 1mL PBS twice, and finally resuspend in 100μl PBS, wash with CD3, CD8 Antibody and CD3, CD4 antibody labeled cells, incubated at 4°C in the dark for 30min. Subsequently, it was fixed with 4% paraformaldehyde for flow detection. Such as Figure 4 As shown in A-4B, VI, pFAu and ARV can effectively induce CD8 + T and CD4 + The content of T cells increased, among which ARV had the strongest induction ability. Serum samples of immunized mice were prepared in the 4th week, and the IgG titer in the serum was determined, such as Figure 4 As shown in C, the prepared ARV has a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com