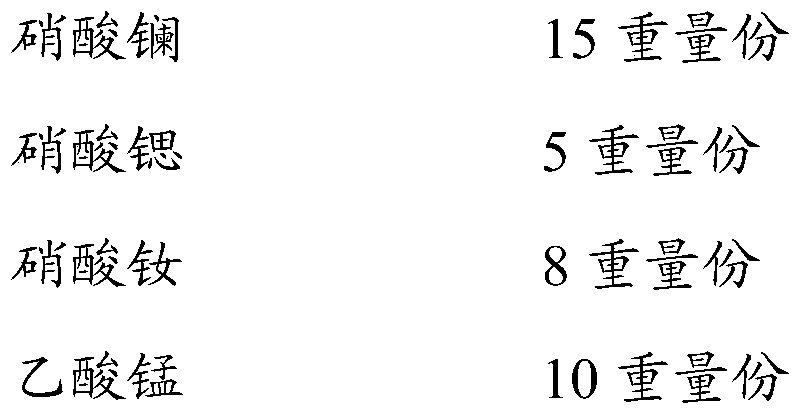Catalyst for synergetic dioxin removal and denitration and preparation method thereof
A technology for removing dioxins and catalysts, which is applied in the field of flue gas removal of dioxins and denitration, can solve the problems of low denitration efficiency, inability to remove dioxins simultaneously and effectively, and easy to be poisoned, so as to achieve efficient removal and improve activity. and stability, prolonging the service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The preparation method of the catalyst for de-dioxin synergistic denitrification provided by the present invention comprises the following steps:
[0028] Step 1, to the lanthanum salt of 10-15 parts by weight, the strontium salt of 5-8 parts by weight, the neodymium salt of 5-8 parts by weight, the manganese salt of 10-15 parts by weight, the chromium salt of 5-8 parts by weight and 5 - Add 18-25 parts by weight of water to 8 parts by weight of iron salt, heat to 40-60 ° C, stir until completely dissolved, then add 20-30 parts by weight of silicon oxide and 4-8 parts by weight of activated carbon powder, and knead Evenly into the first mud;
[0029] Step 2, extruding the first mud material, drying at 20-60°C for 4-6 days, calcining at 500-550°C for 1 day, and then crushing to form a porous powder;
[0030] Step 3, ammonium paramolybdate in 4-7 parts by weight, ammonium metavanadate in 12-18 parts by weight, cobalt salt in 2-5 parts by weight, antimony salt in 1-3 part...
Embodiment 1
[0056] The catalyst for synergistic denitrification of dioxins is prepared by the following components in parts by weight:
[0057]
[0058] Among them, the silicon oxide is nano-state silicon oxide, the size of the nano-grain is 10-20nm, and the activated carbon powder is 2000 mesh powder;
[0059] Titanium dioxide is anatase titanium dioxide, the size of the nano-grain is 8-15nm, and the specific surface area is 90-120m 2 / g;
[0060] The composition of the micro-carbon ferrochrome powder is C≤0.1%, Cr≥60%, Fe balance, and the particle size of the powder is 1500-2000 mesh;
[0061] The diameter of glass fiber filaments is 3-5μm, the length is 1-3mm, and the dispersion degree is above 90%;
[0062] The viscosity-average molecular weights of P.E.O and CMC are 4,000,000 and 20,000, respectively.
[0063] Using the above components to prepare a dioxin-removing synergistic denitrification catalyst through the following steps:
[0064] (1) Add 18 parts by weight of pure wat...
Embodiment 2
[0071] The catalyst for synergistic denitrification of dioxins is prepared by the following components in parts by weight:
[0072]
[0073]
[0074] Among them, the silicon oxide is nano-state silicon oxide, the size of the nano-grain is 10-20nm, and the activated carbon powder is 2000 mesh powder;
[0075] Titanium dioxide is anatase titanium dioxide, the size of the nano-grain is 8-15nm, and the specific surface area is 90-120m 2 / g;
[0076] The composition of the micro-carbon ferrochrome powder is C≤0.1%, Cr≥60%, Fe balance, and the particle size of the powder is 1500-2000 mesh;
[0077] The diameter of glass fiber filaments is 3-5μm, the length is 1-3mm, and the dispersion degree is above 90%;
[0078] The viscosity-average molecular weights of P.E.O and CMC are 6,000,000 and 50,000, respectively.
[0079] Using the above components to prepare a dioxin-removing synergistic denitrification catalyst through the following steps:
[0080] (1) Add 25 parts by weight...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Wire diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com