Diblock fusion protein with adhesion-antifreeze dual functions, and synthesis method and application thereof
A technology of fusion protein and synthesis method, applied in the biological field, can solve the problems of cumbersome preparation method of superhydrophobic material, cumbersome operation, protein inactivation, etc., and achieve the effects of easy later production expansion, low equipment requirements and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Construction of recombinant expression vector and bacterial strain
[0040] ①Construction of recombinant expression vector
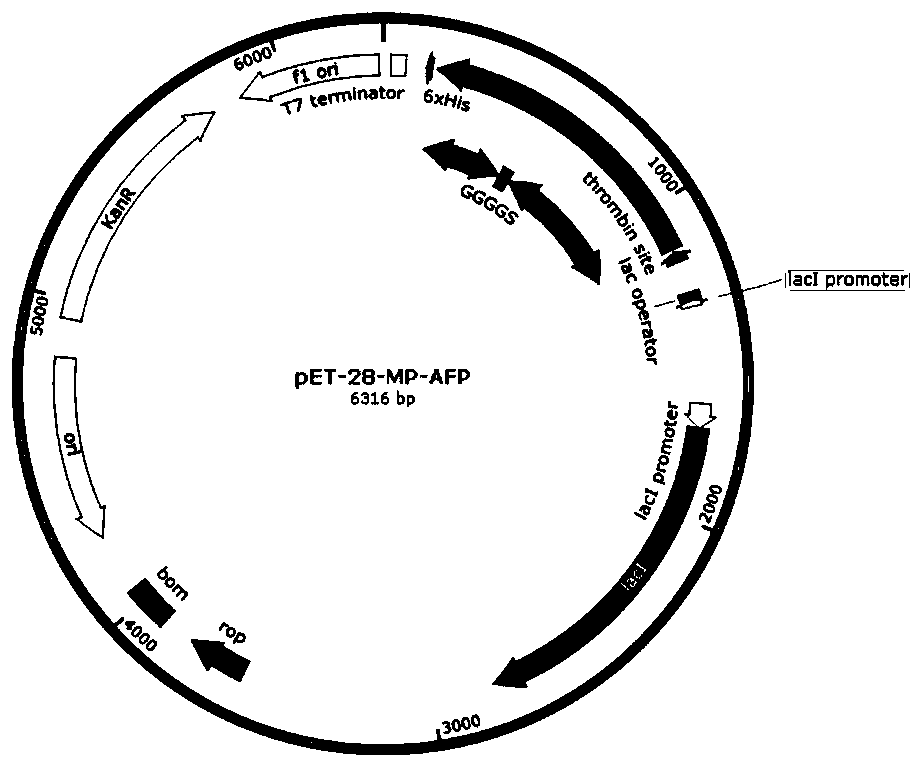
[0041] Three GGGGS repeat sequences were used as the linking polypeptide to connect the adhesion protein and mealworm antifreeze protein to form the fusion protein MP-AFP, and the codon optimization of the gene was carried out according to the expression preference of Escherichia coli without changing its amino acid sequence. A BamHI restriction site was added to the 5' end of the gene sequence and a NotI restriction site was added to the 3' end of the gene; the gene sequence was artificially synthesized and cloned into the E. coli expression vector pET-28a to obtain a recombinant expression vector pET-28a-MP-AFP, the recombinant expression vector contains T7 strong promoter, lca lactose operon, kanamycin resistance marker site and hexahistidine tag, such as figure 1 shown.
[0042] ②Chemical transformation of Escherichia coli
[...
Embodiment 2
[0044] Embodiment 2: Expression and purification of fusion protein MP-AFP
[0045] ① Fermentative expression of fusion protein MP-AFP in Escherichia coli
[0046] The successfully transformed Escherichia coli was streaked and activated on a double-resistant LB solid medium plate containing kanamycin and chloramphenicol. A single colony was picked and placed in a 5 ml test tube containing 50 μg / ml kanamycin and chloramphenicol LB liquid medium, and cultured overnight at 37° C. and 200 rpm with shaking. Transfer the E. coli seed solution in a 1:100 ratio to a 500ml shake flask containing 200ml medium; measure the growth curve of E. coli with a UV spectrophotometer, and when the concentration of the bacteria solution is OD after E. coli transfer 600 When =0.8, 0.8mM IPTG was used to induce Escherichia coli to express the fusion protein MP-AFP, and culture was continued for 12 hours at 37°C and 250rpm, and then the Escherichia coli cells were collected.
[0047] ②Isolation and p...
Embodiment 3
[0054] Embodiment 3: surface modification and atomic force microscope analysis
[0055] ① Surface modification of the sample on the matrix material
[0056] The glass sheet and the mica sheet are used as matrix materials, and water and fusion protein MP-AFP are used as samples to modify the surface of the matrix material. The cleaned matrix material was soaked in the two samples, and incubated at 25° C. with 80% humidity for 12 hours, with at least 3 repetitions for each group. After incubation, the matrix material was washed 3 times with high-purity water to remove excess protein samples.
[0057] ②Atomic force microscope detection
[0058] The surface morphology of the matrix material was detected by the tapping mode of the atomic force microscope, and the surface of the fusion protein-modified glass slide showed a compact and dense protein-modified layer, which was regular and orderly.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com