Application of D609 in preparation of medicine for preventing and treating retinal injury diseases
A technology for retinal damage and retinal pigment, which is applied in the field of biomedicine to achieve strong protective effects, reduce damage, and high efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] (1) method
[0066] 1. Experimental materials and procedures
[0067] 1.1 Cell culture medium preparation: DMEM / F12+10% fetal bovine serum+1% P / S double antibody.
[0068] 1.2 Preparation of various antioxidants: Dilute different antioxidants separately, and use a concentration of 10 μg / mL.
[0069] 1.3 Preparation of sodium iodate: Sodium iodate (SI) was directly diluted with culture medium, and the final concentration was 10 mM.
[0070] 1.4 Treatment of cells:
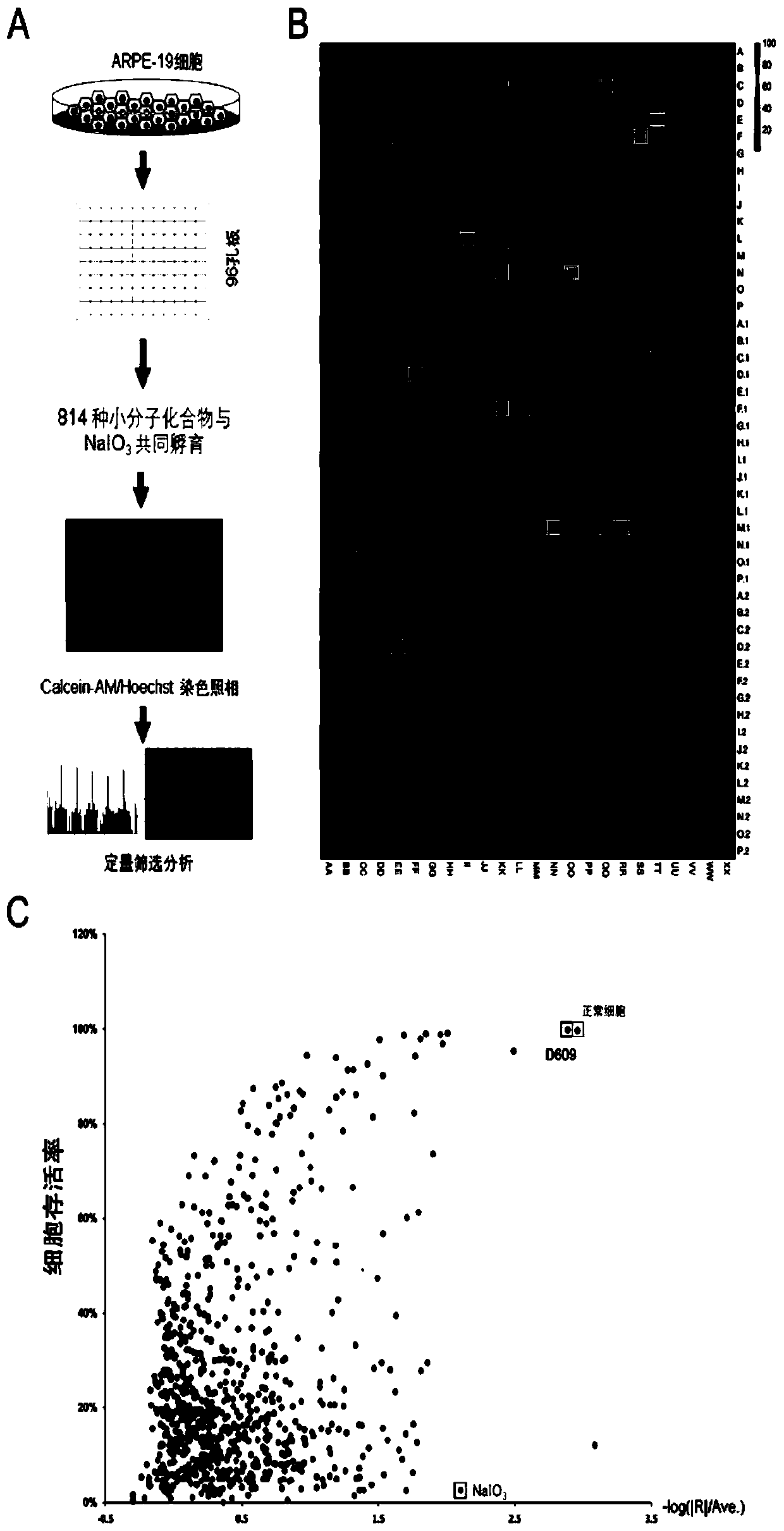
[0071] The retinal pigment epithelial cell line ARPE-19 cells purchased from ATCC will be cultured in the medium of DMEM / F12+10% fetal bovine serum+1% double antibody, and divided into negative control group and model control group (sodium iodate) 1. Experimental group: Sodium iodate + various antioxidants were co-treated separately. Place at 37°C, 5% CO 2 Cultivated in an incubator. Observe cell changes and perform corresponding molecular biology tests.
[0072] (2) Results
[0073] The results are s...
Embodiment 2
[0081] (1) method
[0082] 1. Experimental materials and procedures
[0083] 1.1 Cell culture medium preparation: DMEM / F12+10% fetal bovine serum+1% P / S double antibody
[0084] 1.2 D609 preparation: Dilute D609 powder with ultrapure water, the final concentration is 5mg / mL, and the final use concentration is 10μg / mL.
[0085] 1.3 Preparation of sodium iodate: Sodium iodate (SI) was directly diluted with culture medium, and the final concentration was 10 mM.
[0086] 1.4 Treatment of cells:
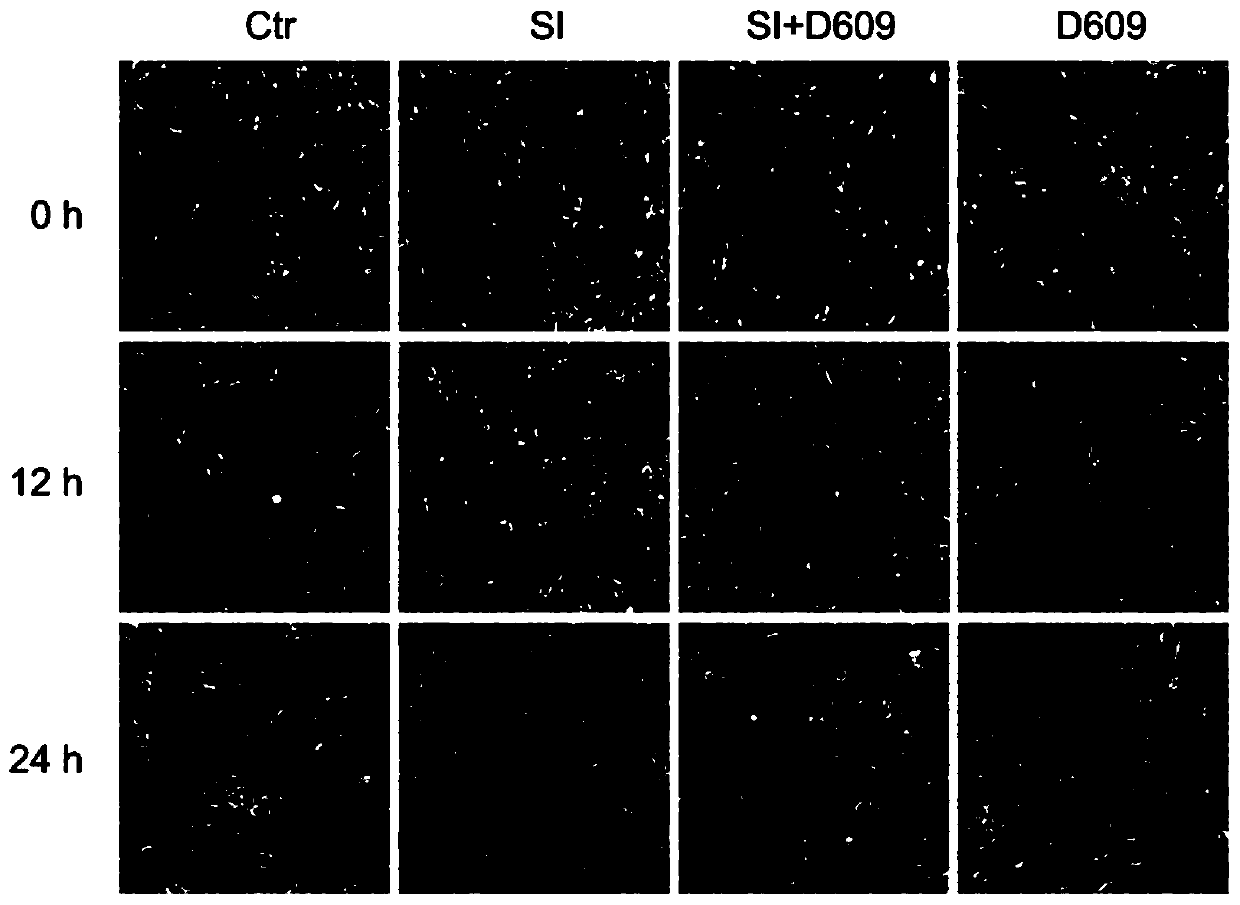
[0087] The retinal pigment epithelial cell line ARPE-19 cells purchased from ATCC will be cultured in the medium of DMEM / F12+10% fetal bovine serum+1% double antibody, and divided into negative control group and model control group (sodium iodate) , Experimental group 1: sodium iodate + D609, experimental group 2: D609. Place at 37°C, 5% CO 2 Cultivated in an incubator. In the experimental group 1, D609 was added to the medium containing 10 mM sodium iodate and diluted to 10 μg / mL, ...
Embodiment 3
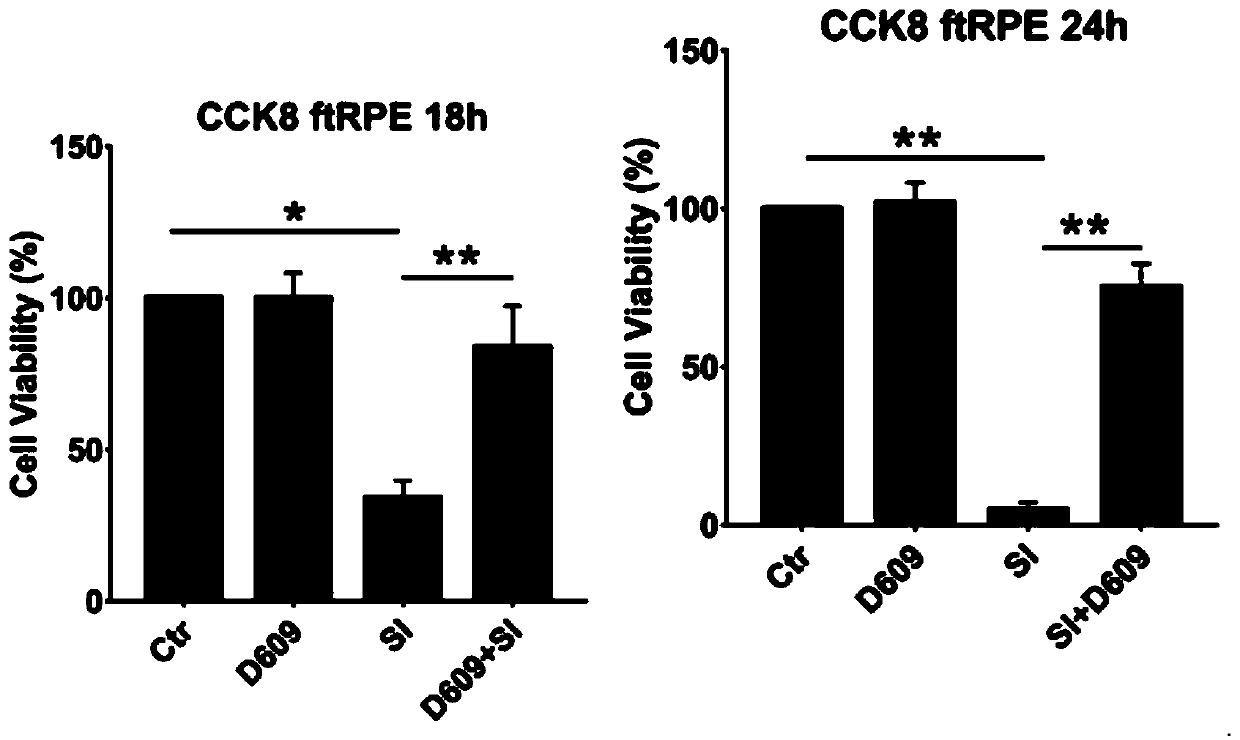
[0094] Example 3 Cell Viability Experiment (CCK8 Staining)
[0095] (1) Method
[0096] 1. Experimental materials and procedures
[0097] 1.1 Cell culture medium preparation: DMEM / F12+10% fetal bovine serum+1% P / S double antibody
[0098] 1.2 D609 preparation: Dilute D609 powder with ultrapure water, the final concentration is 5 mg / mL, and the final use concentration is 10 μg / mL.
[0099] 1.3 Sodium iodate preparation: The sodium iodate powder was directly diluted with culture medium, and the final concentration was 10mM.
[0100] 1.4 Cell preparation: ARPE-19 cells, a retinal pigment epithelial cell line purchased from ATCC, were seeded into 96-well plates, with 10,000 cells per well, and cultured for 48 hours. Divided into negative control group, model control group (sodium iodate), experimental group 1: sodium iodate + D609, experimental group 2: D609. In the experimental group 1, D609 was added to the medium containing 10 mM sodium iodate and diluted to 10 μg / mL, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com