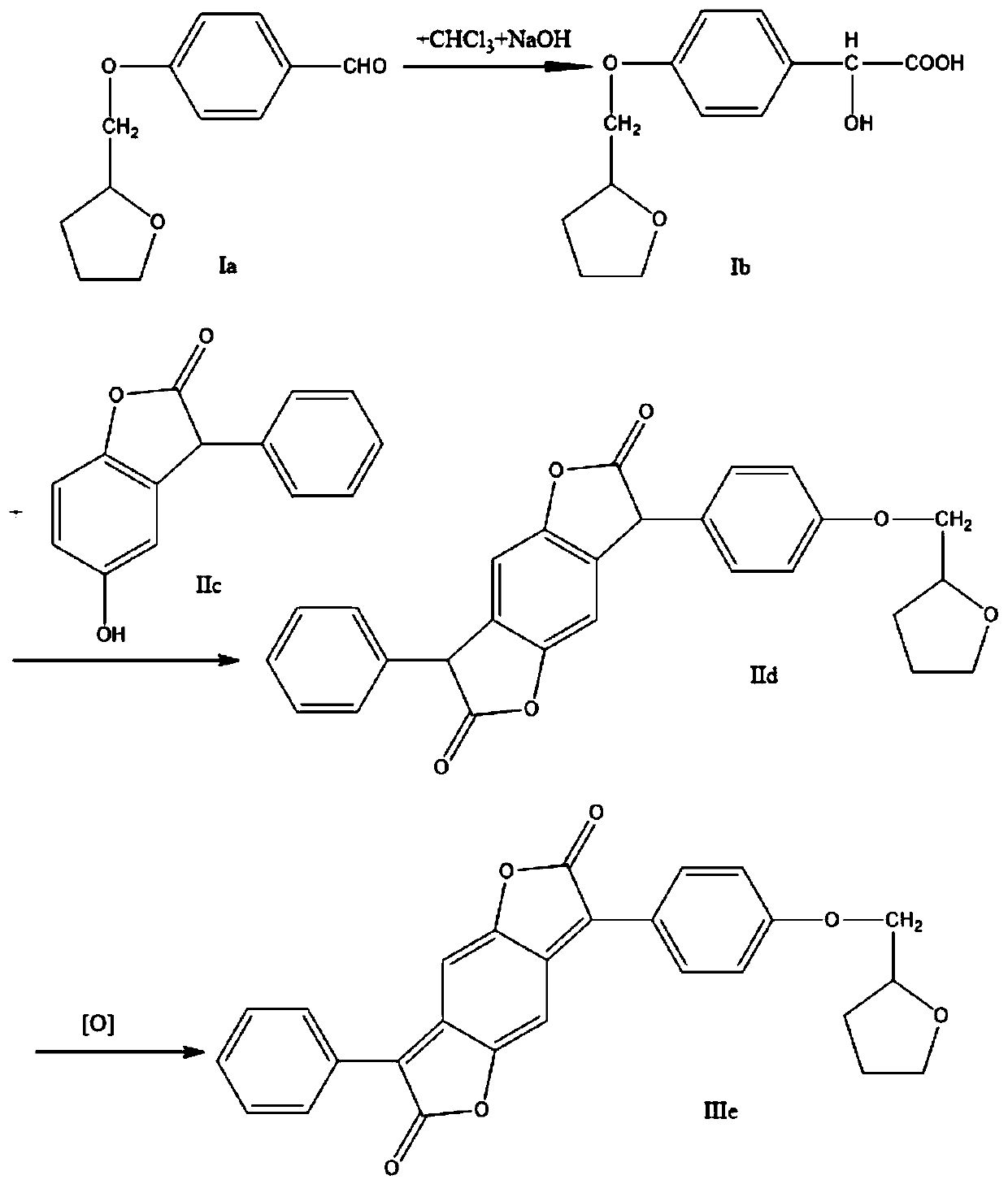
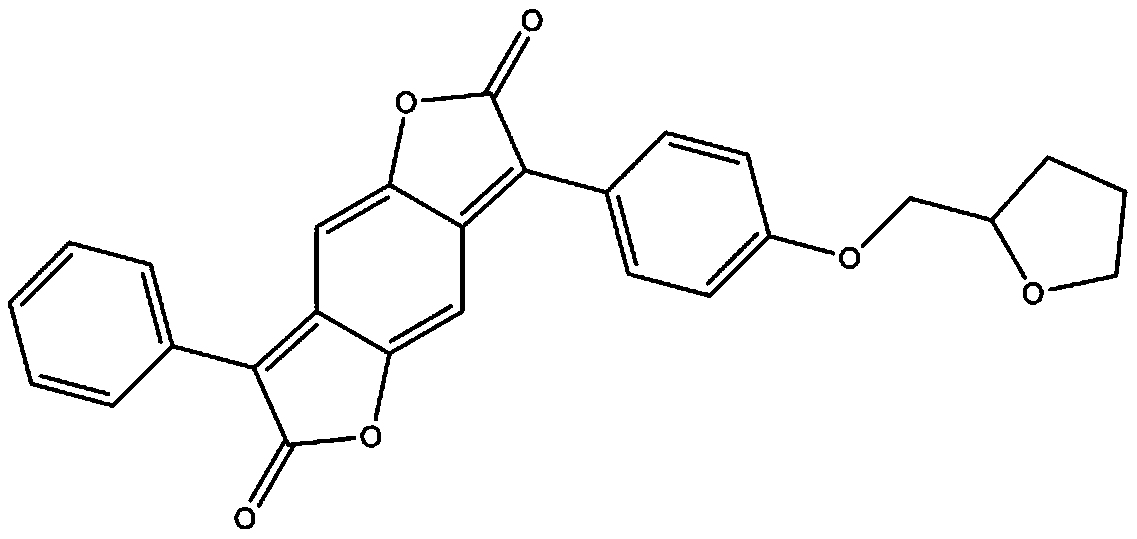
Method for preparing disperse red SBWF
A technology of disperse red and tetrahydrofuran, applied in chemical instruments and methods, azo dyes, organic dyes, etc., can solve the problems of strict operational safety technical requirements, difficult treatment of "three wastes", long process route, etc., and achieve easy control of reactions , Exempt the risk of high-risk operation, the effect of high oxidation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] 1) Add 20.5g of 4-(tetrahydrofuran-2-methoxy)-benzaldehyde, 2g of benzyltriethylammonium chloride, and 49g of chloroform into a three-necked flask in sequence, heat in a water bath, and stir vigorously. The temperature was raised to 50° C., and 33 g of 40% NaOH solution was added dropwise at a rate of 20 seconds per drop. After the dropwise addition was completed, the reaction was continued for 3 hours while maintaining the temperature at 60°C. Follow up the reaction with high performance liquid chromatography, with the content of 4-(tetrahydrofuran-2-methoxyl)-benzaldehyde being lower than 1% as the end point of the reaction, after the reaction is completed, the temperature is naturally lowered, and an appropriate amount of water and chloroform is added to stir, and left to stand for 30 minutes, layered. Separate the organic layer, and use a rotary evaporator to recover chloroform from the organic layer; add 30% hydrochloric acid to the aqueous layer to acidify to pH ...
Embodiment 2
[0019] 1) Add 20.5g of 4-(tetrahydrofuran-2-methoxy)-benzaldehyde, 2.5g of hexadecyltrimethylammonium bromide, and 52g of chloroform into a three-necked flask in sequence, heat in a water bath, and stir vigorously. The temperature was raised to 55° C., and 40 g of 40% NaOH solution was added dropwise at a rate of 20 seconds per drop. After the dropwise addition was completed, the reaction was continued for 3 hours while maintaining the temperature at 60°C. Follow up the reaction with high performance liquid chromatography, with the content of 4-(tetrahydrofuran-2-methoxyl)-benzaldehyde being lower than 1% as the end point of the reaction, after the reaction is completed, the temperature is naturally lowered, and an appropriate amount of water and chloroform is added to stir, and left to stand for 30 minutes, layered. Separate the organic layer, and use a rotary evaporator to recover chloroform from the organic layer; add 30% hydrochloric acid to the aqueous layer to acidify t...
Embodiment 3
[0022] 1) Add 20.5g of 4-(tetrahydrofuran-2-methoxy)-benzaldehyde, 2g of benzyltriethylammonium chloride, and 52g of chloroform into a three-necked flask in sequence, heat in a water bath, and stir vigorously. The temperature was raised to 53° C., and 39 g of 40% NaOH solution was added dropwise at a rate of 20 seconds per drop. After the dropwise addition was completed, the reaction was continued for 3 hours while maintaining the temperature at 60°C. Follow up the reaction with high performance liquid chromatography, with the content of 4-(tetrahydrofuran-2-methoxyl)-benzaldehyde being lower than 1% as the end point of the reaction, after the reaction is completed, the temperature is naturally lowered, and an appropriate amount of water and chloroform is added to stir, and left to stand for 30 minutes, layered. Separate the organic layer, and use a rotary evaporator to recover chloroform from the organic layer; add 30% hydrochloric acid to the aqueous layer to acidify to pH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com