A method for simultaneously preparing two monoclonal antibodies
A monoclonal antibody, hybridoma cell line technology, applied in microorganism-based methods, botanical equipment and methods, biochemical equipment and methods, etc., to achieve the effects of high specificity, low titer, rapid preparation and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]In this example, both Pn33Fps (33F pneumococcal capsular polysaccharide) and HBs (hepatitis B surface protein) are from Yunnan Watson Biotechnology Co., Ltd.; Pn33Fps (10mg) standard was purchased from ATCC; The products were purchased from China National Institutes for Food and Drug Control. BALB / c mice (SPF grade): 6-8 weeks old female, 13-16g, purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. (experimental animal license number: SCXK (Beijing) 2016-0006); SP2 / 0 Myeloma cells were purchased from Kunming Institute of Zoology. Polyethylene glycol 4000 (PEG4000), HAT selection medium (H-Hypoxanthine hypoxanthine, A-Aminopterin aminopterin, T-Thymidine thymidine), Tween-20, and HRP-labeled goat anti-mouse IgG were all purchased From Sigma Company of the United States; RPMI-1640 modified medium (Thermo Fisher, the United States); paraffin oil (Sinopharm Chemical Reagent Co., Ltd., China); fetal bovine serum and neonatal bovine serum were purcha...
Embodiment 2
[0072] The preparation of embodiment 2 monoclonal antibody (mouse ascites)
[0073] 2.1 Pretreatment of mice
[0074] Three BALB / c mice aged 8 to 10 weeks were injected with sterile liquid paraffin, 0.5ml / mouse, and used to inoculate hybridoma cells 7 days later.
[0075] 2.2 Recovery, subcloning and expansion of hybridoma cells
[0076] Resuscitation is carried out according to the existing method. During the culture process, pay attention to observe the number and shape of cells. Subcloning is carried out according to the limited dilution method. After resuscitation, the positive rate of subcloned cells is 100%. Combined with observation under a microscope, select cells with better morphology to proceed. Expand cultivation.
[0077] 2.3 Mice were inoculated with hybridoma cells
[0078] When the cells cover 80% to 90% of the bottom of the plate, blow the cells down, centrifuge at 1200r / min for 5min, discard the supernatant, suspend the cells with RPMI-1640 medium, count t...
Embodiment 3
[0081] The establishment and application of embodiment 3 competition ELISA method
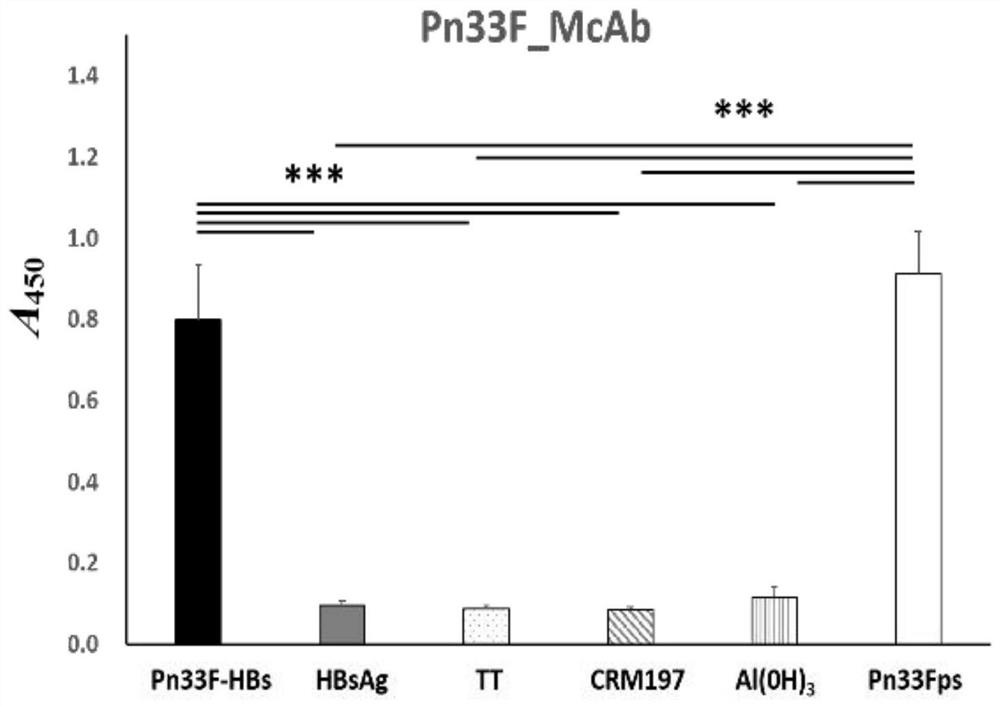
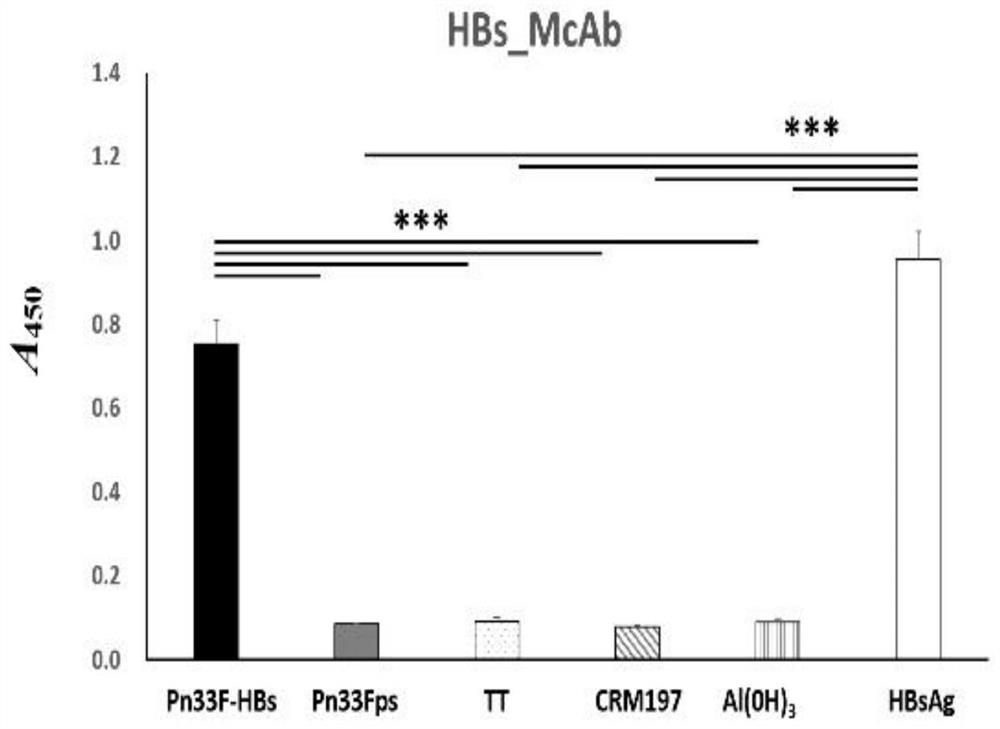
[0082] The indirect ELISA method was used to determine the working concentration of antigen and antibody, namely: Pn33Fps antigen coating concentration was 50ng / 0.1ml per well, antibody dilution was 1:1000; HBsAg antigen coating concentration was 50ng / 0.1ml per well, antibody dilution The degree is 1:2000.
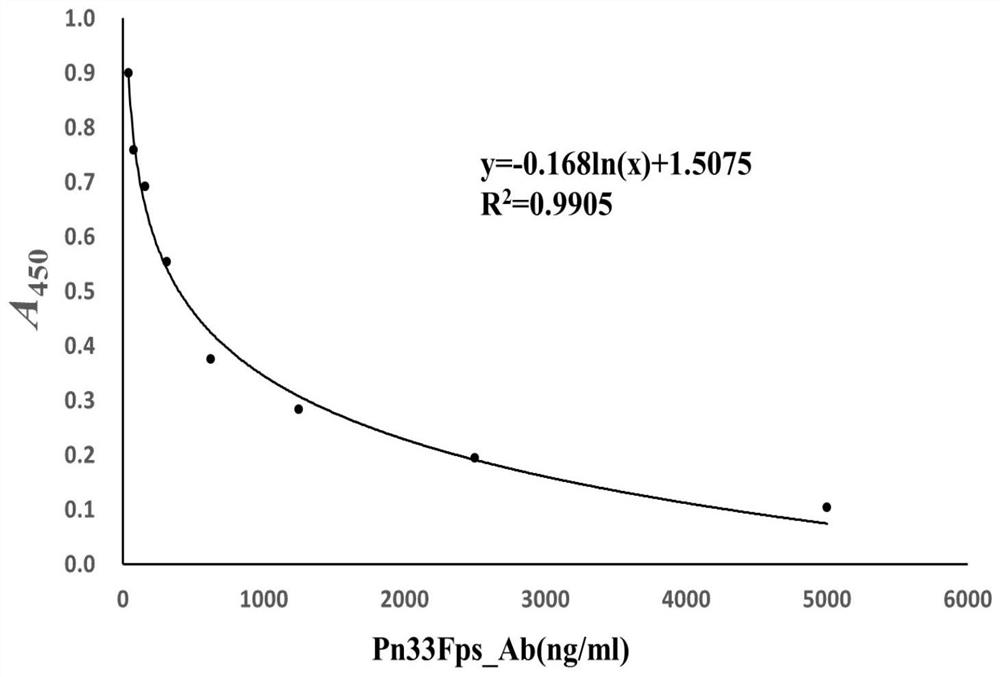
[0083] Use above-mentioned antigen coating concentration and antibody dilution to carry out the detection of competition ELISA, draw Pn33F polysaccharide standard curve (detection range: 40ng-5 μ g, regression equation: y=-0.168ln(x)+1.5075) and HBs antigen standard curve ( Detection range: 8ng-1μg, regression equation: y=-0.128ln(x)+0.9952), the two standard curves show good regression within the detection range, and the regression coefficients are greater than 0.99 ( image 3 and Figure 4 ). Using the same method to detect the Pn33Fps and HBsAg samples of 3 batches prepared in additio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com