Zinc electrode reduction regeneration recycling method of zinc-air battery
A zinc-air battery and zinc electrode technology, which is applied in the field of electrochemical technology and battery manufacturing, can solve the problems of uneven electrolyte flow rate, uneven current distribution, and high cost of electrolytic reduction, so as to prevent zinc oxidation and electrolyte failure, and improve Electric energy utilization rate and the effect of reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
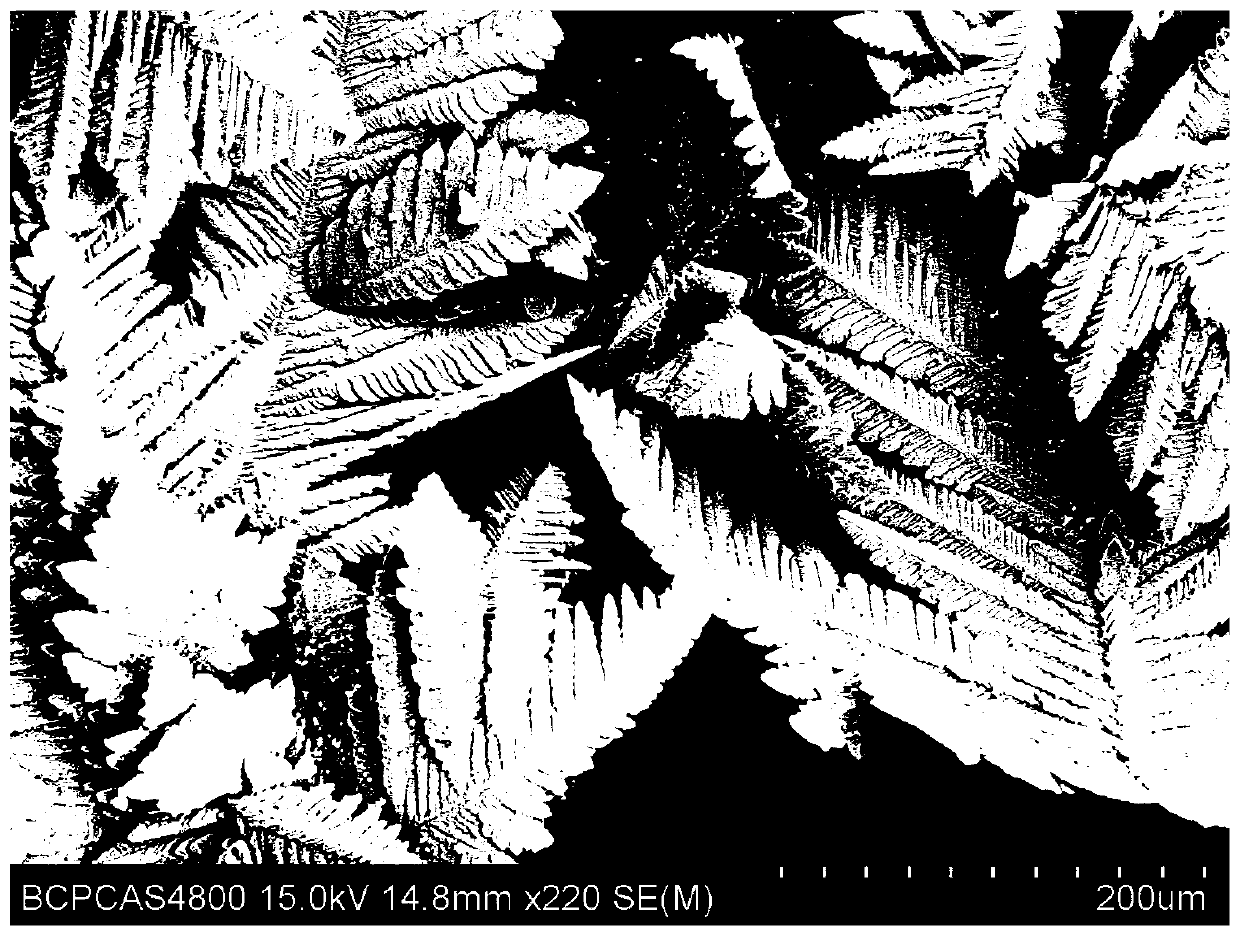
Image
Examples
Embodiment 1
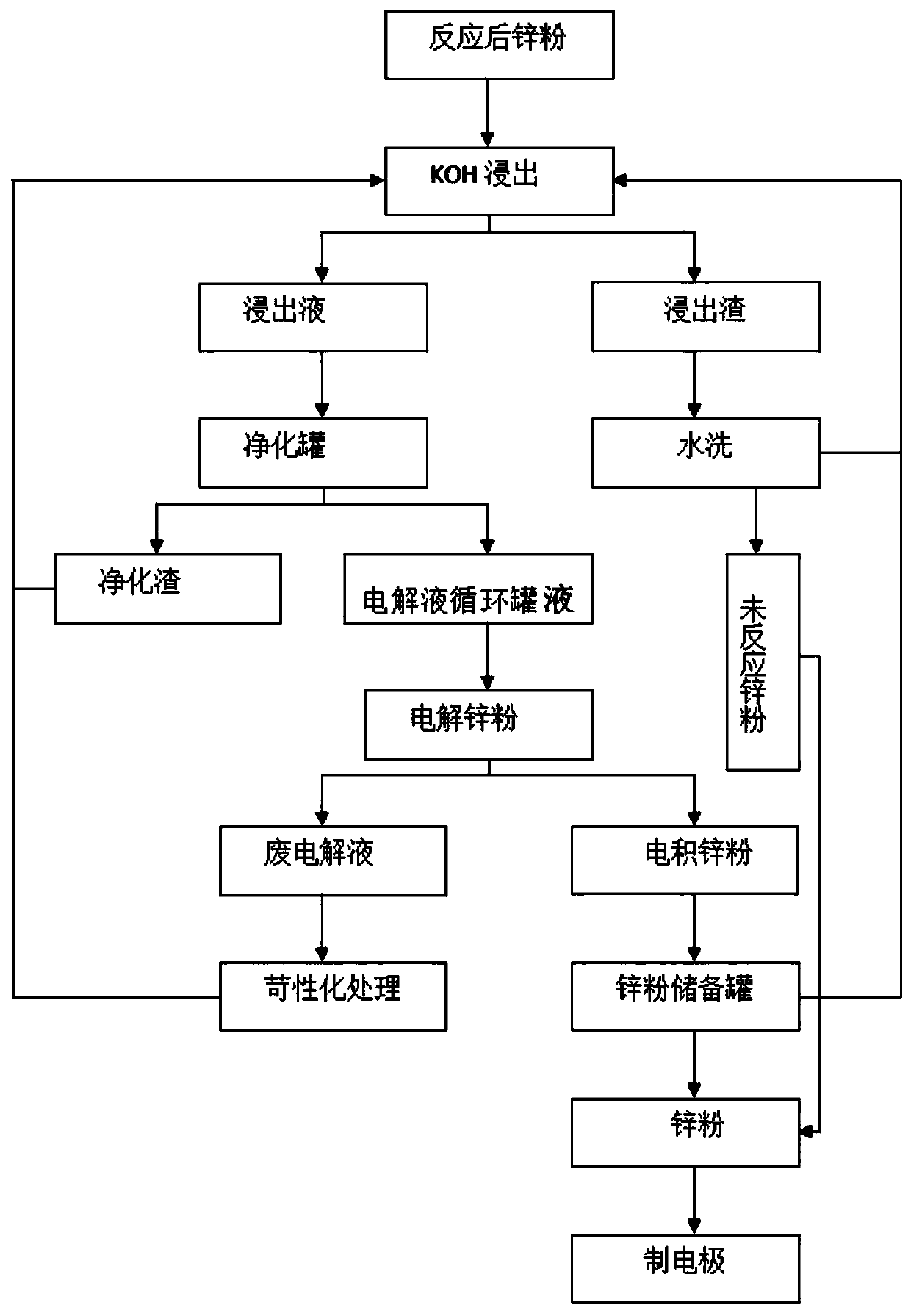
[0026] A zinc-air battery zinc electrode reduction regeneration recycling method is as follows: after the zinc-air battery is discharged, the product is separated from the current collector → enters the alkali solution tank at a specified ratio → filters the alkali solution → separates unreacted zinc for reuse → enters the saturated alkali solution Purification tank→electrolyte circulation tank→electrolyzer→electrolytic reduction of zinc (collecting reaction gas)→zinc collection tank→electrolyte returns to alkali solution tank for recycling; details are as follows:
[0027] S1. Separate the zinc from the current collector, crush the zinc electrode and send it to the constant-temperature stirring alkali-dissolving tank with KOH at a mass ratio of 4:1; the zinc particles in the constant-temperature stirring alkali-dissolving tank are less than 8cm 3 ; KOH alkali concentration 6mol / L; alkali-soluble mass ratio 4:1, temperature 30°C, stirring speed 50 rpm, dissolution time 2h; high...
Embodiment 2
[0030] Embodiment 2: The difference between embodiment 2 and embodiment is that the process parameter is the right end value
Embodiment 3
[0032] S1. Separate the zinc from the current collector, crush the zinc electrode and send it to the constant-temperature stirring alkali-dissolving tank at a mass ratio of 4:1 with KOH; wherein the zinc particles in the constant-temperature stirring alkali-dissolving tank are ≤8cm 3 ; KOH alkali concentration 6mol / L; alkali-soluble mass ratio 4:1, temperature 40°C, stirring speed 50 rpm, dissolution time 3h; high-power DC power supply for electrolytic reduction of metal zinc, which can use solar energy, wind energy, tidal energy, etc. New energy alternative
[0033] S2. After stirring the alkali solution at a constant temperature, perform filtration and separation through a filter separator, and then perform electrolyte purification; the flow rate of the filter separator is controlled to 6m 3 / h, the filter separator separates the solute from the solution when the electrolyte passes through; the unreacted zinc powder is separated, the separated electrolyte is tested, the zinc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com