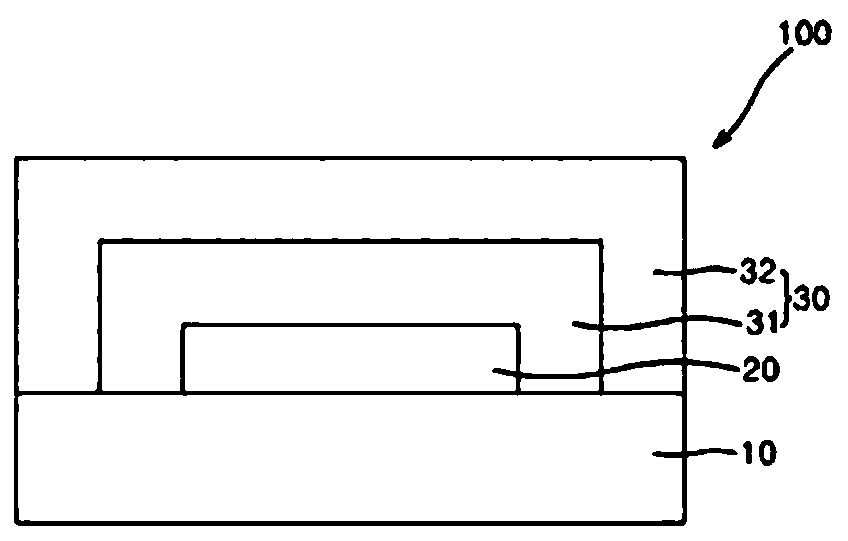
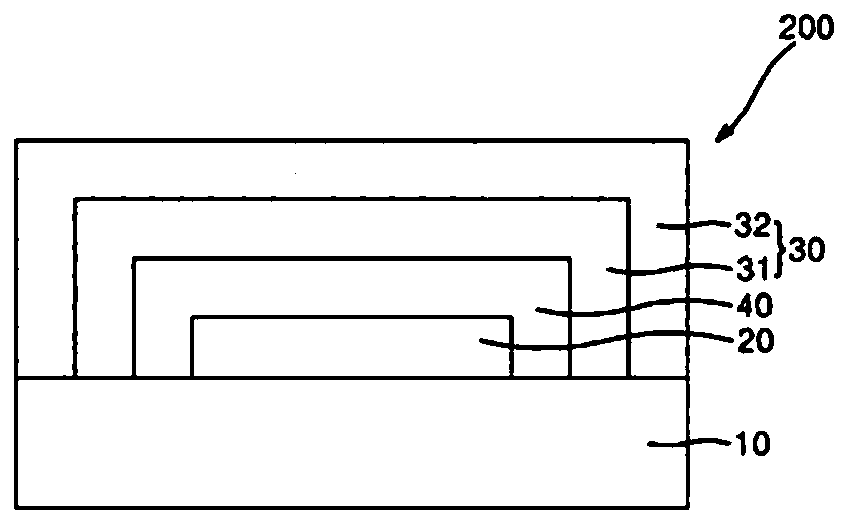
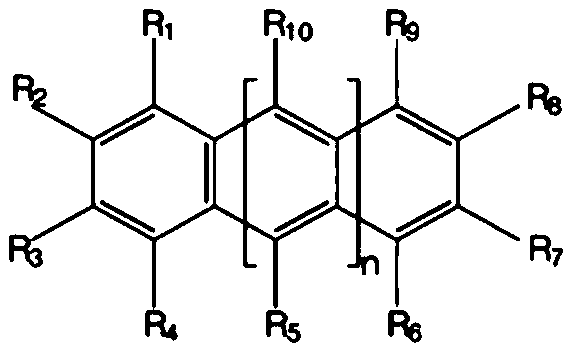
Composition for encapsulating organic light emitting diode and organic light emitting diode display
A technology of light-emitting diodes and compositions, which is applied in the manufacture of electric solid-state devices, semiconductor devices, and semiconductor/solid-state devices, etc., can solve problems such as ultraviolet damage, damage, and shortened lifespan.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0264] Preparation Example 1: Compounds of Preparation Formula 1-4
[0265] In a 1,000ml flask equipped with a cooling tube and a stirrer, place 300ml of acetone, 39g of triethylamine, and 50g of 2,2'-[9,10-anthracenediylbis(oxyl)]bisethanol (2,2 '-[9,10-antracendiylbix(oxy)]bisethanol) (Atomax Co., Ltd.), to which 30.2 g of methyl Acryloyl Chloride. Next, the mixture was stirred for 1 hour after raising the temperature of the flask to 40°C. The remaining solvent was removed by distillation, thereby obtaining the compound of formula 1-4 with a high performance liquid chromatography (HPLC) purity of 96%.
preparation example 2
[0266] Preparation example 2: the compound of preparation formula 3-1
[0267] In a 1,000ml flask equipped with a cooling tube and a stirrer, 200g of cyanoacetic acid, 320g of 2-hydroxyethyl methacrylate, 600ml of toluene, and 3g of concentrated sulfuric acid (Daejung Chemicals & Materials Co., Ltd. ., Ltd.)), then nitrogen was purged for 30 minutes and the flask was heated to 160° C. to remove water therefrom. The solvent was removed by distillation, thereby obtaining 2-acrylate-2-methyl-2-[(cyanocarbonyl)oxy]ethyl ester (2-cyanoacetoxyethyl methacrylate) with an HPLC purity of 96%. ester, weight average molecular weight: 197.19 g / mol). (H NMR ( 1 Hnuclear magnetic resonance, 1H NMR): δ6.12, s, 1H; δ5.62, s, 1H; δ4.45, m, 2H; δ4.38, m, 2H; δ3.01, s, 2H; 3H)
[0268] In the 500ml flask that cooling tube and stirrer are provided, 15.2g KOH, 38g methyl iodide, 50g 2-phenyl-1H-indole-3-formaldehyde and 150g dimethylformamide (dimethylformamide, DMF) are placed and Stirred a...
preparation example 3
[0270] Preparation example 3: the compound of preparation formula 5-2
[0271] In a 1,000ml flask equipped with a cooling tube and a stirrer, 300ml of ethyl acetate, 21g of 3,3-diphenyl-1,1,5,5-tetramethyltrisiloxane and 43g of alkene Propanol (Daei Chemical Materials Co., Ltd.), followed by nitrogen purging for 30 minutes. Next, 72 ppm of Pt-loaded carbon black powder (Aldrich GmbH) was added thereto, and then the flask was heated to 80° C. and the components were stirred for 4 hours. The remaining solvent was removed by distillation, whereby the compound was obtained. 71.5 g of the obtained compound and 39 g of triethylamine were sequentially added to 300 ml of methylene chloride, and then 30.2 g of methacryloyl chloride was slowly added thereto while stirring the mixture at 0°C. The remaining solvent was removed by distillation, thereby obtaining a compound represented by Formula 5-2 with an HPLC purity of 96%. ( 1 H NMR: δ7.52,m,6H; δ7.42,m,4H; δ6.25,d,2H; δ6.02,dd,2H;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com