Novel coumarin two-photon sulfydrylamino acid fluorescent probe
A technology of thiol amino acid and two-photon fluorescence, applied in the field of fluorescent probes, can solve the problems of expensive instruments, complicated operation steps, long response time, etc., and achieve high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The synthesis of embodiment 1 compound I
[0024] Under nitrogen atmosphere and ice bath, 2,4-dinitrobenzenesulfonyl chloride and compound II were added to 20 mL of dry dichloromethane, and triethylamine was added dropwise. After stirring at room temperature overnight, the reaction was completed and concentrated in vacuo, and separated by column chromatography to obtain compound I as a dark red solid. figure 1 is the NMR image of the probe.
Embodiment 2
[0025] The selectivity analysis of embodiment 2 compound I
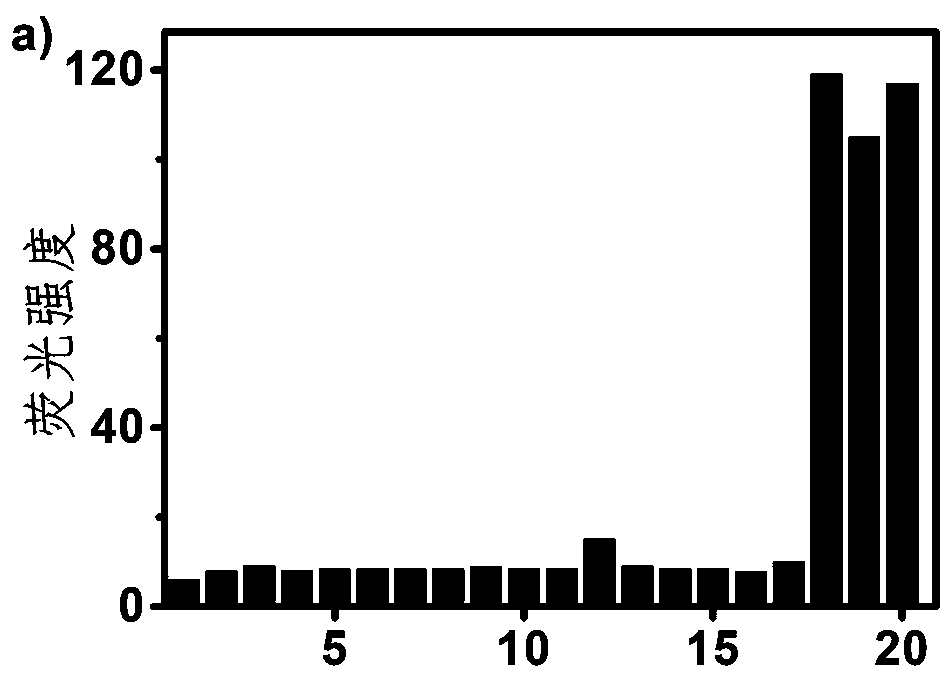
[0026] Add the DMSO solution containing thiol amino acid to the 10 μM compound I DMSO and PBS buffer mixture (volume ratio: 2:98, 1 mM CTAB), so that the final concentration of thiol amino acid is 50 μM, mix After homogenization, the fluorescence intensity change of the solution was detected, and the results were as follows: figure 2 . When the excitation light wavelength is 480nm, the probe compound I has a strong orange fluorescence at 617nm for glutathione, cysteine, and homocysteine, and the probe compound I has no important response to other non-sulfhydryl amino acids . It shows that the probe compound I has excellent selectivity to glutathione, cysteine and homocysteine.
Embodiment 3
[0027] Example 3 Analysis of the response of probe compound I to changes in the concentration of thiol amino acids
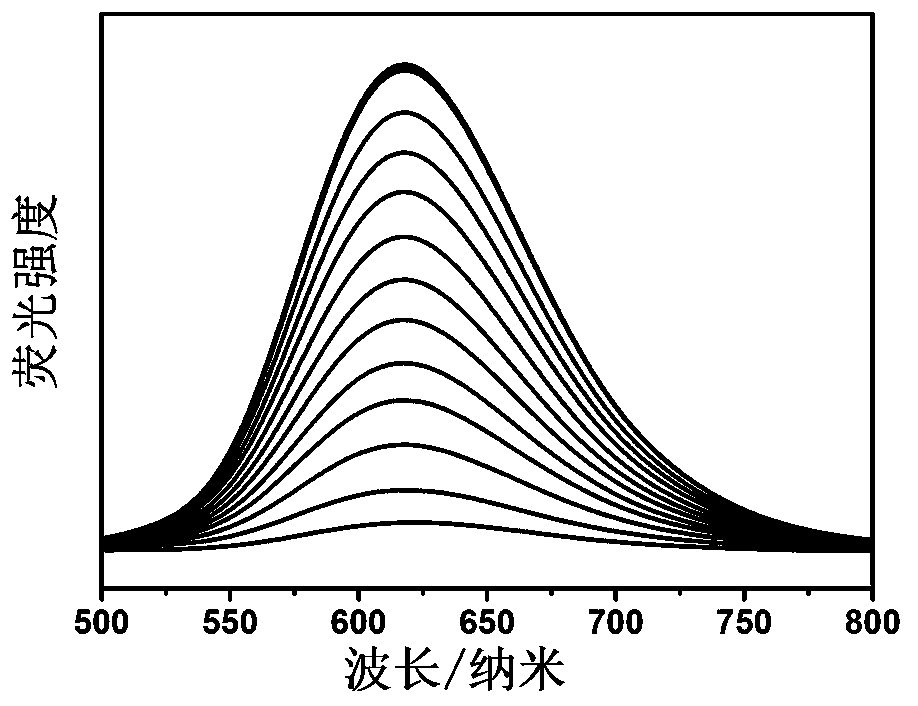
[0028] Add solutions containing different concentrations of glutathione, cysteine, and homocysteine to 10 μM probe compound I in DMSO and PBS buffer mixture (volume ratio 2:98, 1mM CTAB) , the fluorescence intensity at 617nm changes regularly with the increase of glutathione, cysteine and homocysteine, such as image 3 The results shown. The fluorescence intensity was plotted against the concentration, showing a linear relationship, indicating that the probe compound I detects the concentration of various thiol amino acids.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap