Method for efficiently expressing hypersensitive protein by using T4 phage display technology
A phage display and hypersensitive protein technology, applied in the field of high-efficiency expression of hypersensitive proteins, can solve the problems of high cost, complicated operation, and many steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1. Sequence acquisition of harpinEa
[0029] The hrpN sequence of the gene encoding harpinEa protein derived from the strain Erwinia amylovora has been published (GeneBank number: M92994.3). The harpinEa gene can be obtained by using well-known technologies in the industry, such as gene synthesis, PCR amplification, extraction of chromosomal DNA, and establishment of a library. The present invention adopts a gene synthesis method to send the amino acid sequence of hrapinEa for synthesis (Suzhou Jinweizhi Biotechnology Company, China).
Embodiment 2
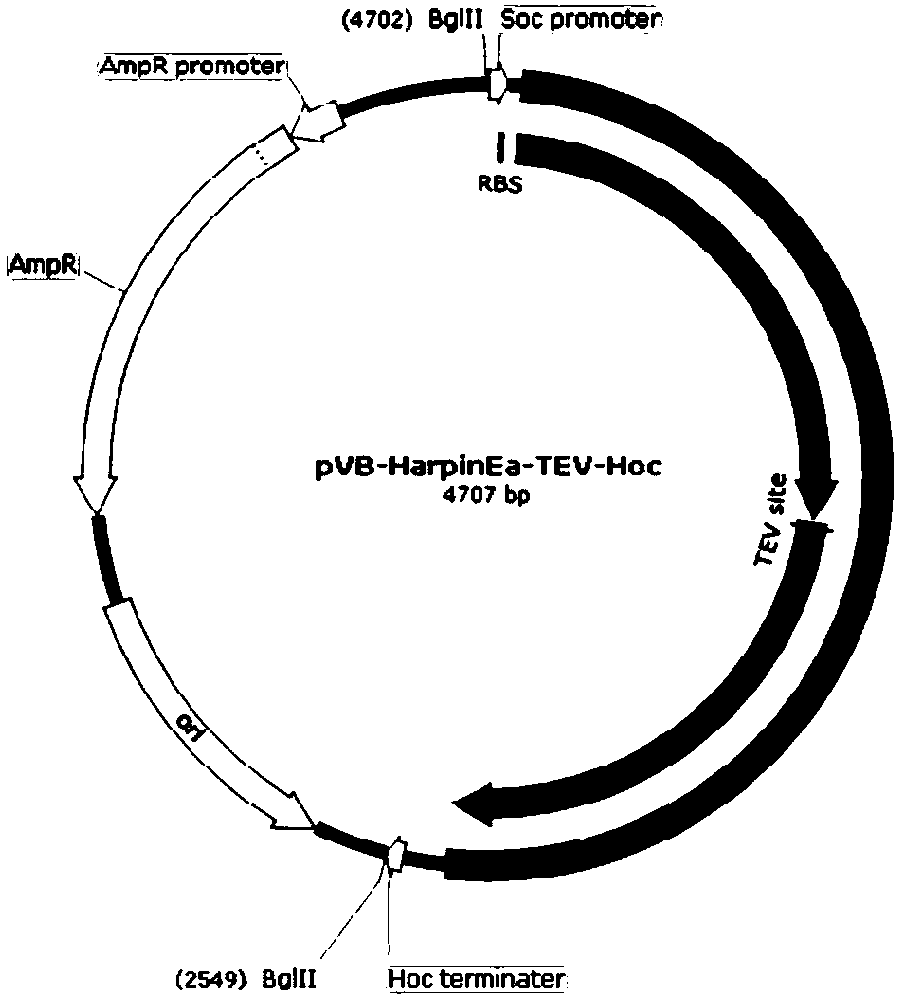
[0030] Example 2. Construction of pVB-harpinEa-TEV-Hoc plasmid
[0031] The backbone of the plasmid is derived from the pUC18 plasmid. Using the pUC18 plasmid as a template, KOD high-fidelity DNA polymerase was used to amplify the ampicillin resistance gene and plasmid replication region by PCR. The PCR forward primer is the 18 bases in the upstream sequence of the pUC18 ampicillin resistance gene, and the reverse primer is the reverse complement of the 18 bases in the downstream sequence of the pUC18 plasmid replication region. Both the forward and reverse primers have a BglII restriction site added to the 5'ends. At the same time, we used T4 phage DNA as a template and used specific primers to amplify Hoc protein gene fragments by PCR. The 5'end of the forward primer introduced the gene sequence of TEV protease. Then, by bridge PCR, the synthesized harpinEa protein gene fragment and the TEV-Hoc protein gene fragment are fused into a DNA molecule, where the coding region of th...
Embodiment 3
[0033] Example 3. In vivo expression and phage display of fusion recombinant protein
[0034] The T4 phage synchronous display expression plasmid pVB-harpinEa-TEV-Hoc was transformed into competent cells of Escherichia coli P301 strain by chemical transformation method, and cultured to bacteria under the pressure of ampicillin antibiotics in a shaker at 37°C at 200 rpm Density reaches 3×10 8 Pcs / ml. Hoc deleted with Hoc protein - T4 bacteriophage (given by the laboratory of Professor Rao, Department of Biology, Catholic University of America) infects the cultured E. coli cells at MOI (multiplicity of infection)=1, and continues to incubate in a shaker at 37°C at 200 rpm for 30 min. During this process, the harpinEa-TEV-Hoc recombinant protein is expressed under the control of the T4 phage particle assembly pathway and displayed on the capsid.
[0035] After the display is completed, the culture solution is immediately aliquoted in pre-cooled centrifuge tubes, and centrifuged at 43...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com