Application of oleanane triterpenoid compound in resisting hepatitis B virus
A technology of oleanane and triterpenoids, applied in the application field of oleanane triterpenoids in anti-hepatitis B virus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
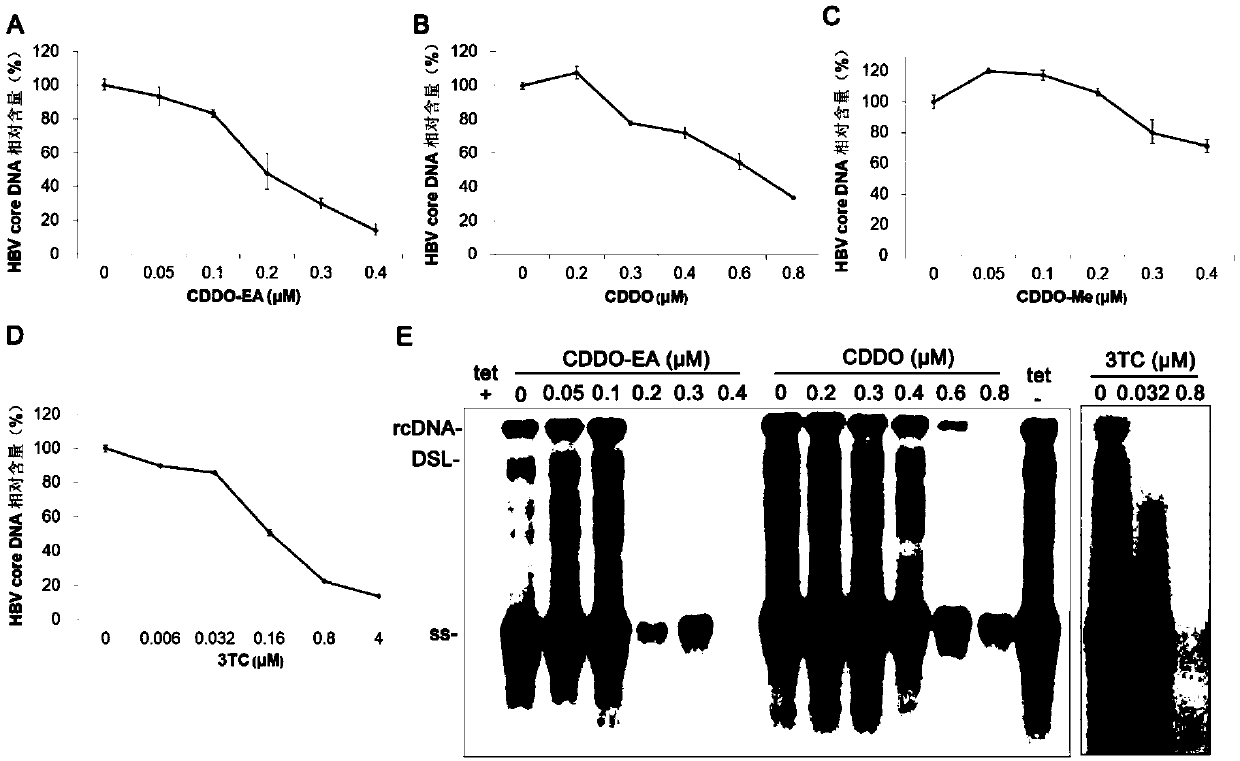
[0049] Example 1 The inhibitory effect of oleanane triterpenoids on HBV DNA
[0050] 1. Cell culture
[0051] HepAD38 cell subculture medium (tet+): containing 10% fetal bovine serum (Gbico), 380 μg / ml G418 (Gibco), penicillin and streptomycin double antibody 100 U / ml (Gibco) and 2 μg / mL tetracycline (Tetracycline, tet , Sigma) MEM (Gibco) medium.
[0052] Culture medium (tet-) used for HepGAD38 cell plate / dilution drug: containing 10% fetal bovine serum (Gbico), 380 μg / ml G418 (Gibco), penicillin and streptomycin double antibody 100U / ml (Gibco).
[0053] When the confluence of HepAD38 cells reaches 90%, add 0.25% trypsin-EDTA (Gibco) to the culture flask, digest at 37°C for 5 minutes, discard the trypsin, continue to digest the residual solution at 37°C for 5 minutes, add complete culture solution containing tetracycline Blow off, 1:3 passaging, passaging once every 3-4 days.
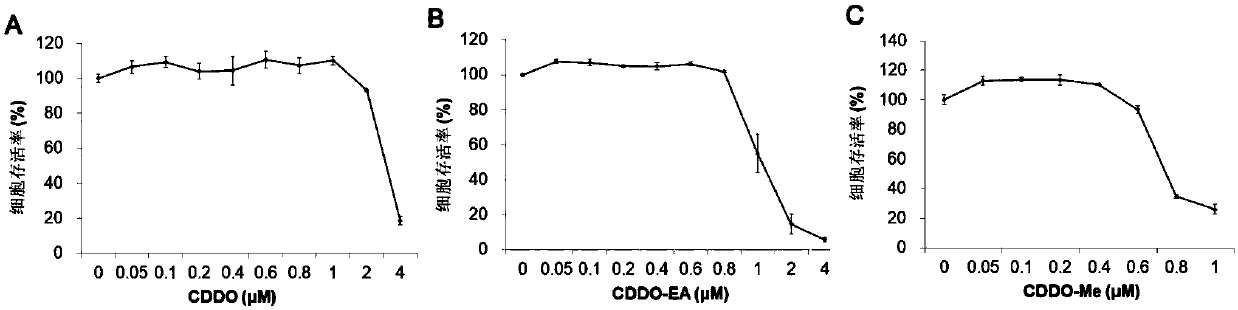
[0054] 2. Cytotoxicity detection
[0055] HepAD38 cells were seeded in 96-well plates, 2×10 4 ...
Embodiment 2
[0086] Example 2 The inhibitory effect of oleanane triterpenoids on HBV pgRNA
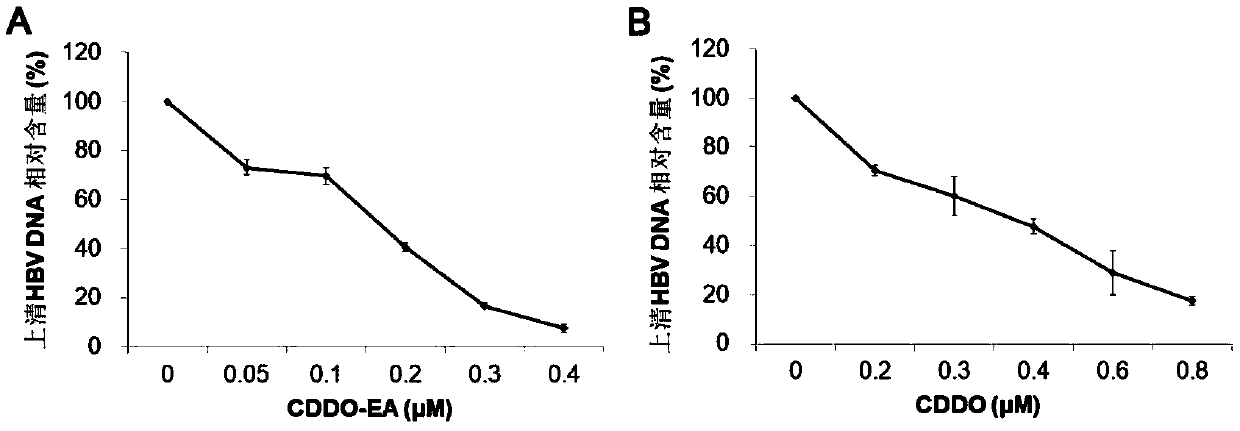
[0087] HepAD38 cells were seeded in 24-well plates, 1×10 5 Each well was cultured at 37° C. and 5% CO2; after 24 hours, the culture medium in the well plate was discarded, and different concentrations of drugs diluted with complete medium without tetracycline were added. Set up a cell control group (culture medium containing tetracycline and no drug, tet+), a virus control group (culture medium without tetracycline and no drug, tet-) and an experimental drug group (CDDO and CDDO-EA). The same culture medium was replaced once on the 3rd day after adding the drug, and the supernatant liquid was discarded 6 days after the drug was added, and the 24-well plate containing the cells was stored at -80°C for testing.
[0088] 1.1 Total HBV pgRNA in cells
[0089] Intracellular total RNA extraction: add TRIzol (Invitrogen) to 24-well plate, 1 mL / well, lyse on ice for 10 min; add 200 μL chloroform for extr...
Embodiment 3
[0106] Example 3 Effect of oleanane triterpenoids on HBc protein content
[0107] CDDO and CDDO-EA acted on HepAD38 cells with 4 μM 3TC (does not affect HBc and capsid assembly), 2 μM Bay41-4109 (purchased from MedChemExpress Company, reduces HBc content and degrades capsid protein) and 5 μM BA-38017 (Baruch, USA) .Professor Guo Jutao from S Blumberg Institute donated it as a control drug, and the sample collection process was the same as in Example 2.
[0108] Total cell protein lysate preparation (5×): 0.05% bromophenol blue (mass / volume ratio), 0.3M Tris-HCl (pH=6.8), 50% glycerol (volume ratio), 10% SDS (mass / volume ratio ), 25% β-mercaptoethanol (volume ratio).
[0109] Add 100 μL of 1× protein lysate to the 24-well plate cells, lyse on ice for 10 minutes, transfer to EP tube;
[0110] Electrophoresis: prepare stacking gel (concentration is 5%) and separating gel (concentration is 10%), sample carries out SDS-PAGE electrophoresis, adopts voltage 60V in stacking gel, aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com