Inactivated vaccine for epizootic haematopoietic necrosis disease in carassius auratus and preparation method of inactivated vaccine for epizootic haematopoietic necrosis disease in carassius auratus
A technology of hematopoietic organ necrosis and inactivated vaccine is applied in the field of crucian carp hematopoietic necrosis disease inactivated vaccine and its preparation field, which can solve the problems of economic loss of crucian carp breeding industry, threat to the healthy development of the industry, strong virus infectivity, etc., and achieve immune protection. Good effect, low production cost, good safety performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The present embodiment provides a preparation method of crucian carp hematopoietic necrosis inactivated vaccine, comprising the following steps:
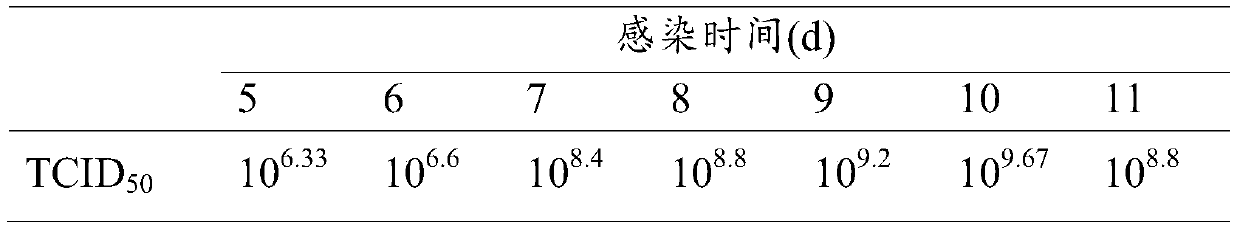
[0024] Step 1, CSC cell culture: take out the spinal cord tissue of heterogeneous gibel crucian carp under aseptic conditions, and process it into a size of 30-60mm 3 Put the tissue block into a culture dish containing L15 culture medium; put the tissue block evenly into a T25 cell culture bottle with the side of the tissue block facing up, add 3ml of L15 culture solution to the culture bottle, overnight, slowly Turn the culture bottle sideways to soak the tissue block in the culture medium, and then turn the side with the tissue block upwards, operate once from time to time until CSC cells grow on the edge of the tissue block, and culture the cell culture bottle upright, every 2 to 3 Replace the culture medium once a day; take 1 bottle of CSC covered with a single layer, discard the old medium in a sterile ultra-clean bench,...
Embodiment 2
[0033] Determination of CyHV-2 inactivation conditions and safety test.
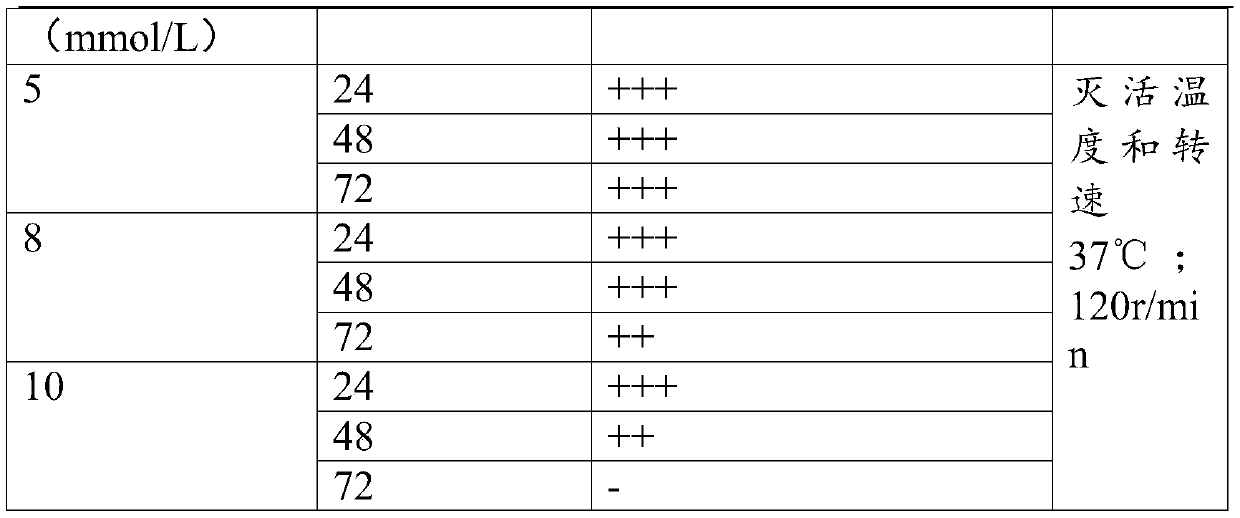
[0034] Obtain the amplified virus stock solution according to the method of Example 1, divide it into 3 equal parts, add BEI to the final concentrations of BEI respectively 5mmol / L, 8mmol / L, and 10mmol / L, and inactivate it in a constant temperature shaker at 37°C at 120r / min After 24h, 48h, and 72h, use the same concentration of sodium thiosulfate solution to terminate the inactivation and take samples. Take the prepared vaccine and inoculate CSC cells according to the above virus propagation method, and set up a negative control at the same time, observe for 7 to 10 days, if the cytopathic effect appears, it indicates that the virus inactivation is not complete; if no cytopathic effect is seen, then blindly pass 2 Second, if cytopathy occurs in the blind transmission, it indicates that the virus inactivation is still incomplete. If no cytopathy occurs in the two blind transmissions, it indicates that th...
Embodiment 3
[0041] Safety Test of Inactivated Vaccines
[0042] Sterility test: Take the vaccine prepared in Example 1, inoculate the brain infusion bacterial medium (BHI) plate, apply the streak method on the plate, and cultivate it at 30°C for 15 days. If there is bacterial colony growth, it indicates that the vaccine has bacterial contamination. ; if no colonies grow, the vaccine is sterile.
[0043] Fish body safety test: Take the vaccine prepared above and inject 30 healthy crucian carp of about 300g, the injection dose is 0.2-0.4ml / tail, and the negative control is injected with the same dose of normal saline. After feeding for 15 to 30 days, if the vaccine group died or had clinical symptoms, but the negative control group did not show clinical symptoms or died, it indicated that the vaccine was unsafe; if neither the vaccine group nor the negative control group showed clinical symptoms or died, it indicated that the vaccine Safety.
[0044] Stress test and food intake effect aft...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com