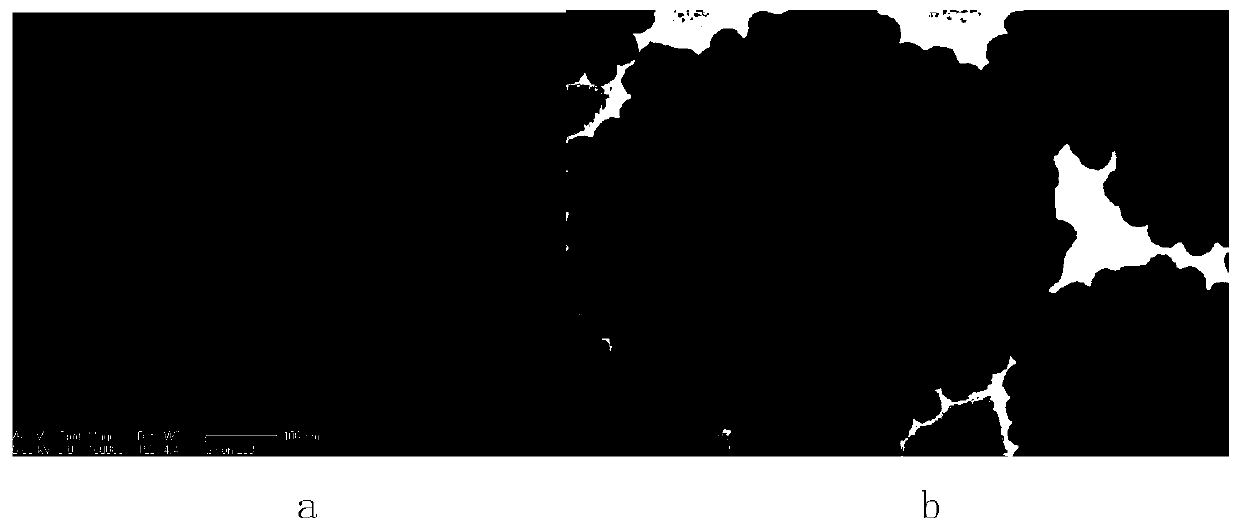
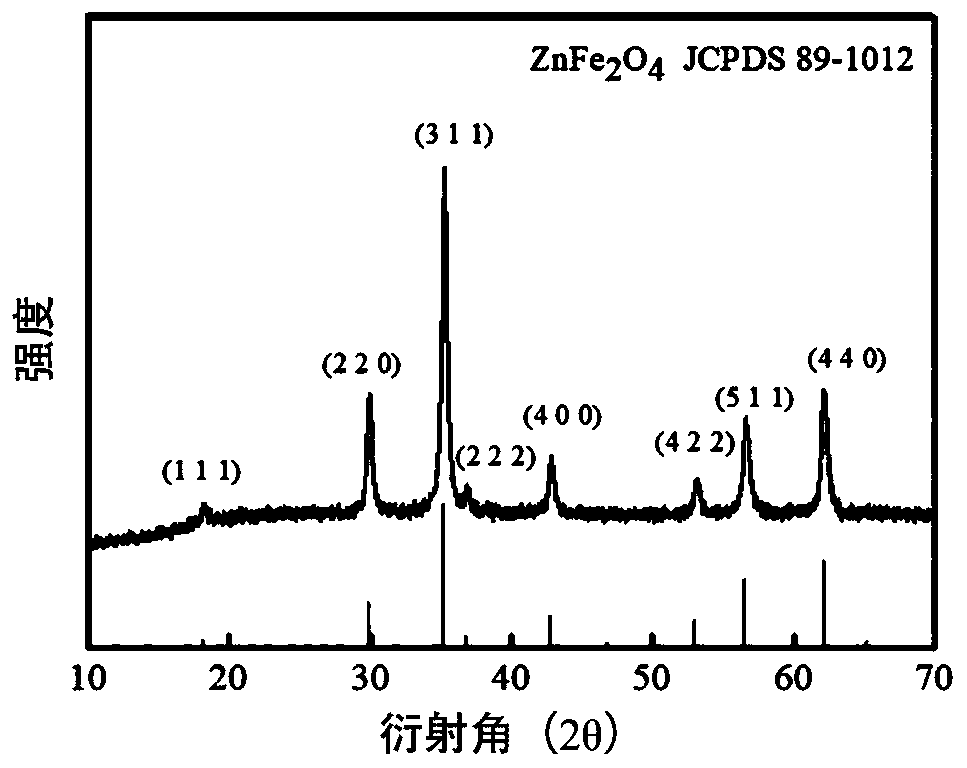
Zinc ferrite hollow sphere with micro-nano structure and preparation method thereof
A micro-nano structure, zinc ferrite technology, applied in chemical instruments and methods, nanotechnology, nanotechnology, etc., can solve the problems of inability to obtain products with uniform size, specific surface area, uneven product size, and small specific surface area of products, etc. To achieve the effect of few kinds of raw materials, uniform size and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The concrete steps of preparation are:
[0034] Step 1, respectively prepare 2wt% urea aqueous solution, 3wt% trisodium citrate dihydrate aqueous solution, 0.6wt% ferric chloride hexahydrate aqueous solution and 0.8wt% zinc nitrate aqueous solution.
[0035] Step 2, according to the volume ratio of the above-mentioned urea aqueous solution, trisodium citrate dihydrate aqueous solution, ferric chloride hexahydrate aqueous solution and zinc nitrate aqueous solution is 0.8:1.2:0.8:2 ratio, first urea aqueous solution and trisodium citrate dihydrate The sodium aqueous solution is uniformly mixed to obtain a mixed solution; then the ferric chloride hexahydrate aqueous solution and the zinc nitrate aqueous solution are sequentially added to the mixed solution and mixed uniformly to obtain a precursor solution.
[0036] In step 3, first place the precursor solution at 120° C. for a closed reaction for 16 hours to obtain a reaction solution. Then the reaction solution is subje...
Embodiment 2
[0038] The concrete steps of preparation are:
[0039] Step 1: prepare 2.25wt% urea aqueous solution, 2.75wt% trisodium citrate dihydrate aqueous solution, 0.95wt% ferric chloride hexahydrate aqueous solution and 0.65wt% zinc nitrate aqueous solution respectively.
[0040] Step 2, according to the volume ratio of the above-mentioned urea aqueous solution, trisodium citrate dihydrate aqueous solution, ferric chloride hexahydrate aqueous solution and zinc nitrate aqueous solution is 0.9:1.1:0.9:1.9 ratio, first urea aqueous solution and dihydrate citrate trihydrate The sodium aqueous solution is uniformly mixed to obtain a mixed solution; then the ferric chloride hexahydrate aqueous solution and the zinc nitrate aqueous solution are sequentially added to the mixed solution and mixed uniformly to obtain a precursor solution.
[0041] In step 3, first place the precursor solution at 135° C. for a closed reaction for 15 hours to obtain a reaction solution. Then the reaction soluti...
Embodiment 3
[0043] The concrete steps of preparation are:
[0044] Step 1, respectively prepare 2.5wt% urea aqueous solution, 2.5wt% trisodium citrate dihydrate aqueous solution, 1.3wt% ferric chloride hexahydrate aqueous solution and 0.5wt% zinc nitrate aqueous solution.
[0045]Step 2, according to the volume ratio of the above-mentioned urea aqueous solution, trisodium citrate dihydrate aqueous solution, ferric chloride hexahydrate aqueous solution and zinc nitrate aqueous solution is 1:1:1:1.8 ratio, first urea aqueous solution and trisodium citrate dihydrate The sodium aqueous solution is uniformly mixed to obtain a mixed solution; then the ferric chloride hexahydrate aqueous solution and the zinc nitrate aqueous solution are sequentially added to the mixed solution and mixed uniformly to obtain a precursor solution.
[0046] In step 3, first place the precursor solution at 150° C. for a closed reaction for 14 hours to obtain a reaction solution. Then the reaction solution is subjec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com