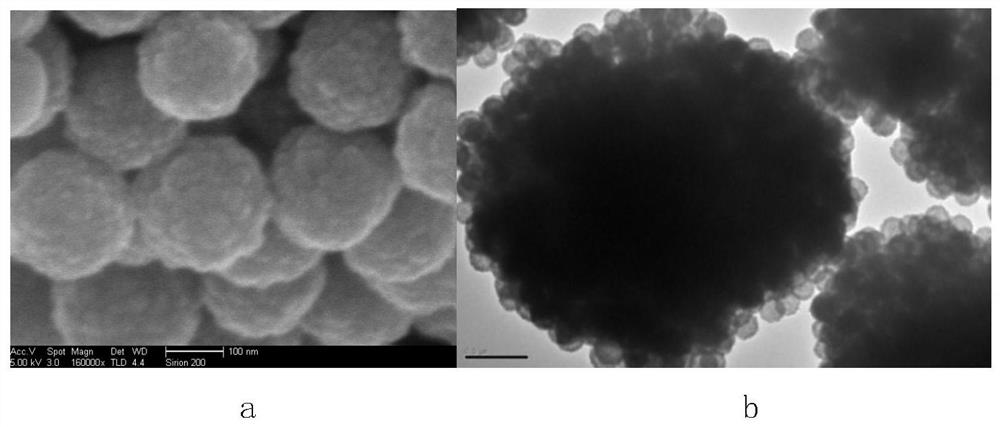
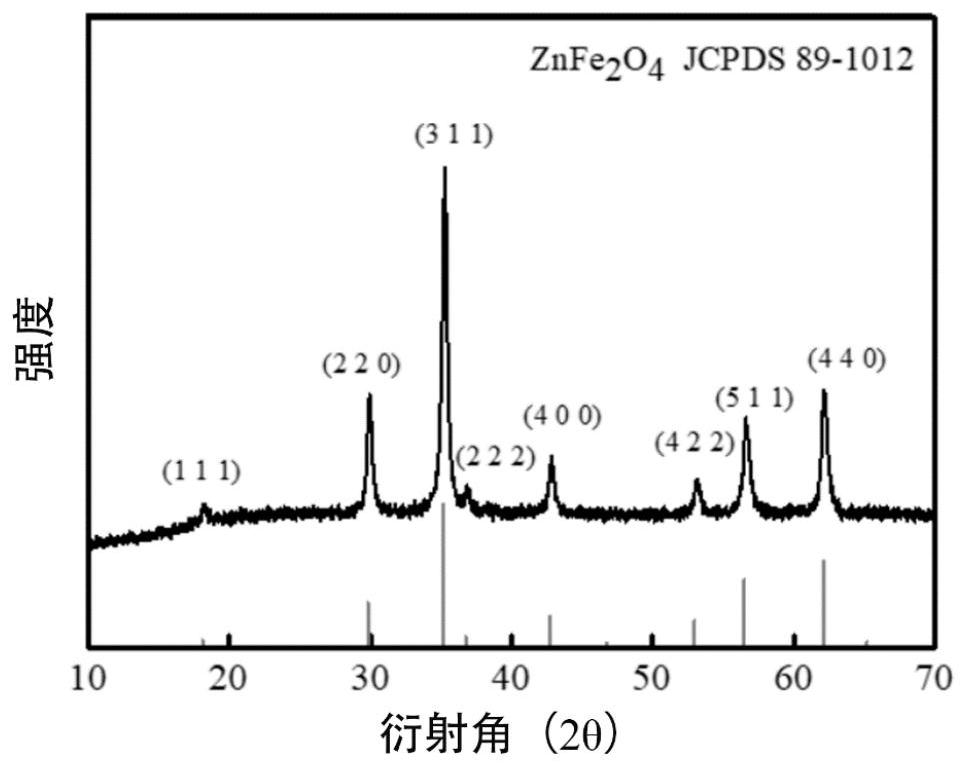
Zinc ferrite hollow sphere with micro-nano structure and preparation method thereof
A technology of micro-nano structure and zinc ferrite, applied in chemical instruments and methods, iron compounds, nanotechnology, etc., can solve the problems of not being able to obtain products with uniform size, specific surface area, restricting the scope of application, and uneven product size. Achieve the effect of fewer types of raw materials, easy separation, and uniform size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The specific steps of preparation are:
[0034] Step 1, respectively preparing 2wt% urea aqueous solution, 3wt% trisodium citrate dihydrate aqueous solution, 0.6wt% ferric chloride hexahydrate aqueous solution and 0.8wt% zinc nitrate aqueous solution.
[0035] Step 2, according to the volume ratio of the above-mentioned urea aqueous solution, trisodium citrate dihydrate aqueous solution, ferric chloride hexahydrate aqueous solution and zinc nitrate aqueous solution to a ratio of 0.8:1.2:0.8:2, first mix the urea aqueous solution and dihydrate citric acid trisodium The sodium aqueous solution is mixed evenly to obtain a mixed solution; and then the ferric chloride hexahydrate aqueous solution and the zinc nitrate aqueous solution are added to the mixed solution and mixed uniformly to obtain a precursor solution.
[0036] In step 3, the precursor solution was placed at 120° C. for a closed reaction for 16 hours to obtain a reaction solution. The reaction solution is then...
Embodiment 2
[0038] The specific steps of preparation are:
[0039] Step 1, respectively preparing 2.25wt% urea aqueous solution, 2.75wt% trisodium citrate dihydrate aqueous solution, 0.95wt% ferric chloride hexahydrate aqueous solution and 0.65wt% zinc nitrate aqueous solution.
[0040] Step 2, according to the volume ratio of the above-mentioned urea aqueous solution, trisodium citrate dihydrate aqueous solution, ferric chloride hexahydrate aqueous solution and zinc nitrate aqueous solution to a ratio of 0.9:1.1:0.9:1.9, first mix the urea aqueous solution and dihydrate citric acid trisodium The sodium aqueous solution is mixed evenly to obtain a mixed solution; and then the ferric chloride hexahydrate aqueous solution and the zinc nitrate aqueous solution are added to the mixed solution and mixed uniformly to obtain a precursor solution.
[0041] In step 3, the precursor solution was placed at 135° C. for a closed reaction for 15 hours to obtain a reaction solution. The reaction soluti...
Embodiment 3
[0043] The specific steps of preparation are:
[0044] Step 1, respectively preparing 2.5wt% urea aqueous solution, 2.5wt% trisodium citrate dihydrate aqueous solution, 1.3wt% ferric chloride hexahydrate aqueous solution and 0.5wt% zinc nitrate aqueous solution.
[0045]Step 2, according to the volume ratio of the above-mentioned urea aqueous solution, trisodium citrate dihydrate aqueous solution, ferric chloride hexahydrate aqueous solution and zinc nitrate aqueous solution to a ratio of 1:1:1:1.8, first mix the urea aqueous solution and dihydrate citric acid trisodium The sodium aqueous solution is mixed evenly to obtain a mixed solution; and then the ferric chloride hexahydrate aqueous solution and the zinc nitrate aqueous solution are added to the mixed solution and mixed uniformly to obtain a precursor solution.
[0046] In step 3, the precursor solution was placed at 150° C. for a closed reaction for 14 hours to obtain a reaction solution. The reaction solution is then ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com