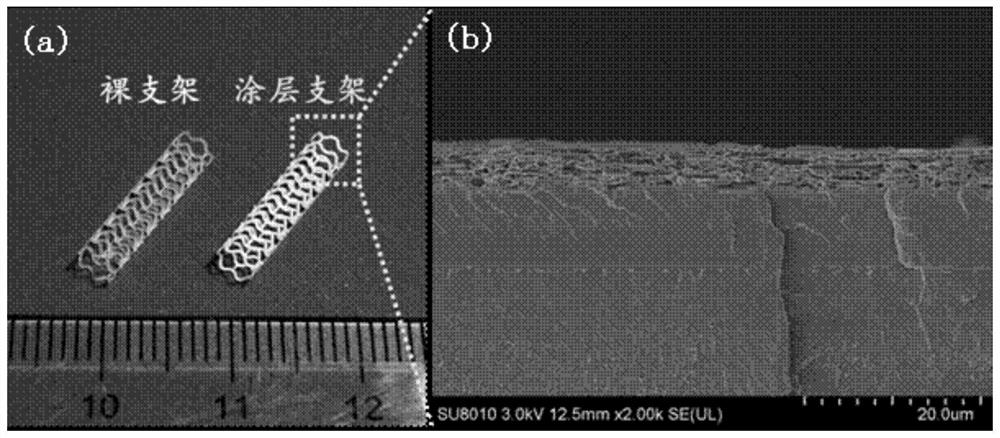
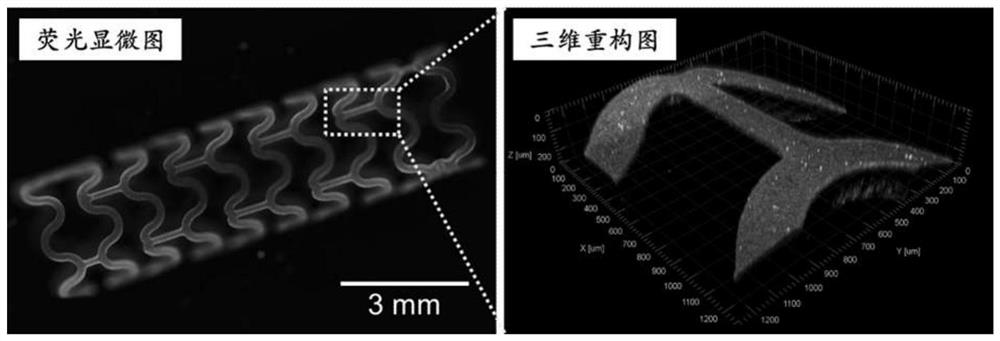
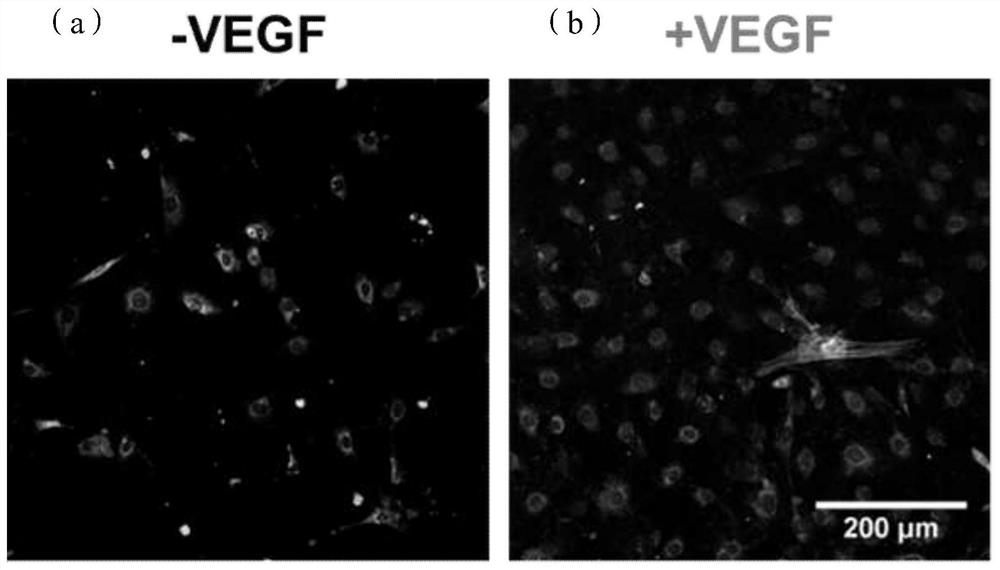
A kind of cardiovascular stent coating based on double-layer heterogeneous structure and preparation method thereof
A cardiovascular and out-of-phase technology, applied in the field of cardiovascular stent coating and its preparation based on double-layer heterogeneous structure, can solve the problems of increasing the risk of late thrombosis, accelerating the repair of endothelial layer, and destroying bioactive molecules. Risk of small late thrombosis, inhibition of intimal hyperplasia, effect of promoting intimal hyperplasia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: VEGF / rapamycin co-loaded stent coating
[0047] (1) Preparation of rapamycin / PDLLA drug layer: PDLLA with a number average molecular weight of 100,000 was used as a degradable coating material, rapamycin was used as an anti-proliferative drug, and chloroform was used as a solvent, prepared by ultrasonic atomization spraying The drug-loaded base layer has a drug coating thickness of 10 μm, and when the base is a stent, the rapamycin content is 6 μg / mm.
[0048] (2) Preparation of heparinized PDLLA porous coating: PDLLA with a double bond-terminated number average molecular weight of 100,000 is used as the coating material, polyvinylpyrrolidone (PVP) with a weight average molecular weight of 40,000 is used as the dissolution phase, and the mass fraction of the dissolution phase was 55%, using chloroform as a solvent, the blend was coated on the base drug-loaded layer by ultrasonic spraying method, and a porous coating with a thickness of 5 μm was obtained by st...
Embodiment 2
[0053] Example 2: VEGF / paclitaxel co-loaded stent coating
[0054] (1) Preparation of paclitaxel / PLGA drug layer: PLGA (LA:GA=75:25) with a number average molecular weight of 100,000 was used as a degradable coating material, paclitaxel was used as an anti-proliferation drug, and chloroform was used as a solvent. For the drug base layer, the thickness of the drug coating is 10 μm, and the drug loading is 5 μg / mm.
[0055] (2) Preparation of hyaluronic acid-modified PLGA porous coating: PLGA with a double bond-terminated number average molecular weight of 100,000 is used as the coating material, and polyvinylpyrrolidone (PVP) with a weight average molecular weight of 40,000 is used as the dissolution phase. The mass fraction is 60%, chloroform is used as a solvent, the blend is coated on the substrate drug-loaded layer by ultrasonic spraying method, and a porous coating with a thickness of 5 μm is obtained by sterile ultrapure water dissolution. Subsequently, the surface of th...
Embodiment 3
[0058] Example 3: VEGF / zotarolimus co-loaded stent coating
[0059] (1) Preparation of zotarolimus / PDLLA drug layer: PDLLA with a number average molecular weight of 150,000 was used as a degradable coating material, paclitaxel was used as an anti-proliferation drug, and chloroform was used as a solvent. The drug-loaded base layer was prepared by ultrasonic spraying. The layer thickness was 5 μm, and the drug loading was 5 μg / mm.
[0060] (2) Preparation of heparinized PLGA porous coating: PDLLA with a double bond-terminated number average molecular weight of 100,000 is used as the coating material, polyethylene glycol (PEG) with a weight average molecular weight of 10,000 is used as the dissolution phase, and the mass fraction of the dissolution phase is 50%, chloroform was used as a solvent, and the blend was coated on the substrate drug-loaded layer by ultrasonic spraying method, and a porous coating with a thickness of 5 μm was obtained by eroding with sterile ultrapure wat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com