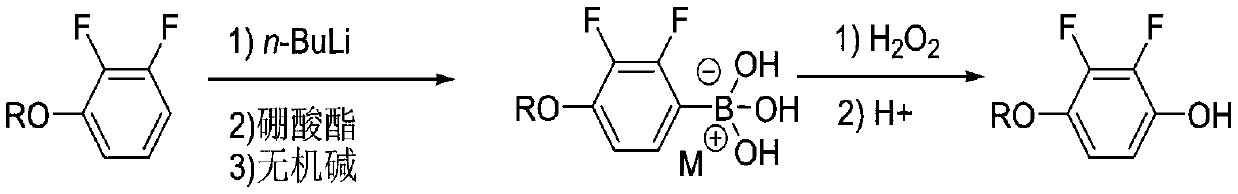
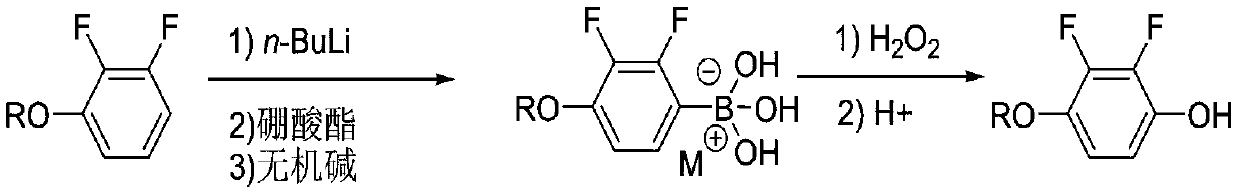
Synthesis process of 2, 3-difluoro-4-alkoxy phenol
A technique for the synthesis of alkoxyphenols, which is applied in the field of synthesis techniques of 2,3-difluoro-4-alkoxyphenols, can solve the problems of difficulty in purifying qualified products, low yields, and poor reaction effects, and achieve The effect of low cost, low equipment requirements and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Step 1: 1000mL four-neck bottle, equipped with a thermometer and a condenser tube, under nitrogen protection, put 57.6g (0.4mol) of 2,3-difluoroanisole and 440mL of tetrahydrofuran, start stirring, and cool down to -85°C in a low-temperature bath At ~-75°C, 192 mL (0.48 mol) of n-butyl lithium in n-hexane solution (2.5 mol / L) was slowly added dropwise. After the addition was complete, the mixture was stirred at controlled temperature for 2.0 hours. After the heat preservation is completed, 62.3 g (0.6 mol) of trimethyl borate is started to be added dropwise at a controlled temperature of -85°C to -75°C, and the dropwise addition is completed and the temperature is kept for 2.0 hours. The residual raw material of 2,3-difluoroanisole detected by liquid phase detection in the sample delivery was less than 0.5%.
[0028] After the reaction is complete, negative pressure precipitates tetrahydrofuran until the temperature of the reaction bottle reaches 50°C and there is no fl...
Embodiment 2
[0031] The first step: 1000mL four-neck bottle, equipped with a thermometer and a condenser, under the protection of nitrogen, put 63.3g (0.4mol) of 2,3-difluorophenethyl ether and 480mL of tetrahydrofuran, start stirring, and cool down to -85°C in a low-temperature bath At -75°C, 192 mL (0.48 mol) of n-butyl lithium in n-hexane (2.5 mol / L) was slowly added dropwise, and after the addition was complete, the mixture was stirred under temperature control for 2.0 hours. After the heat preservation is completed, 87.6 g (0.6 mol) of triethyl borate is started to be added dropwise at a controlled temperature of -85°C to -75°C, and the dropwise addition is completed and the temperature is kept for 2.0 hours. The residual raw material of 2,3-difluorophenetole was detected by liquid phase detection <0.5%.
[0032] After the reaction is complete, negative pressure precipitates tetrahydrofuran until the temperature of the reaction bottle reaches 50°C and there is no flow, stop the precip...
Embodiment 3
[0035] The first step: 1000mL four-neck bottle, equipped with a thermometer and a condenser, under the protection of nitrogen, put 63.3g (0.4mol) of 2,3-difluorophenetole and 480mL of tetrahydrofuran, start stirring, and cool down to -70°C in a low-temperature bath At -60°C, 192 mL (0.48 mol) of a cyclohexane solution of n-butyllithium (2.5 mol / L) was slowly added dropwise. After the addition was complete, the mixture was stirred at a controlled temperature for 2.0 hours. After the heat preservation is completed, 112.9 g (0.6 mol) of isopropyl borate is started to be added dropwise at a controlled temperature of -70° C. to -60° C., and the dropwise addition is completed and the temperature is kept for 2.0 hours. The residual raw material of 2,3-difluorophenetole was detected by liquid phase detection in sample delivery <0.5%.
[0036]After the reaction is complete, negative pressure precipitates tetrahydrofuran until the temperature of the reaction bottle reaches 50°C and ther...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com