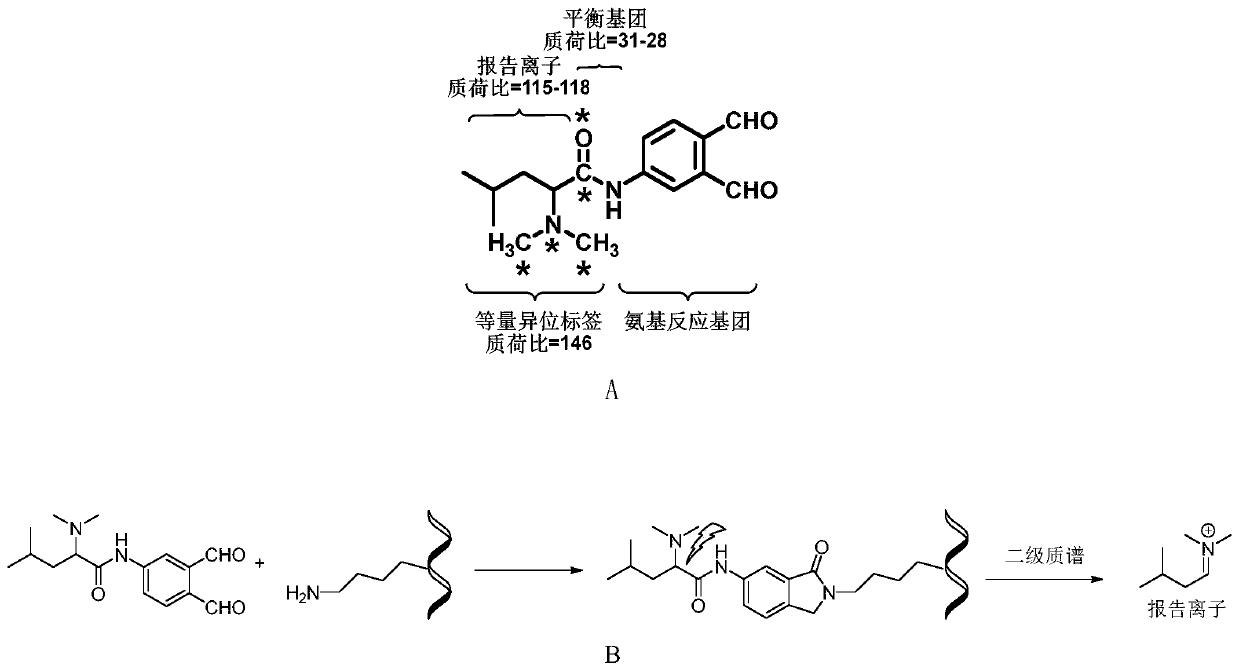
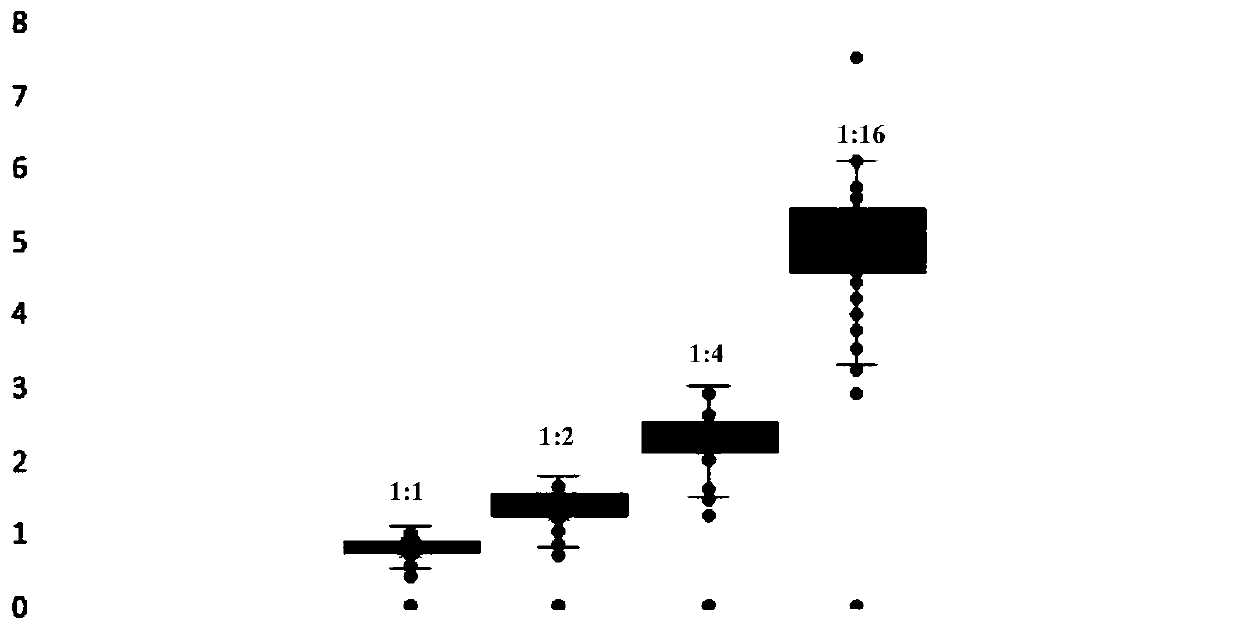
Protein quantitative labeling reagent and preparation method and application thereof
A technology for labeling reagents and proteins, applied in the field of proteomics research, can solve problems such as easy hydrolysis, reduced ionization efficiency of mass spectrometry, and low labeling efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] The preparation of embodiment 1 formula 2 compound
[0060] In this embodiment, step 1 of the above-mentioned method is adopted, and the reduction reaction is carried out with 4-nitrophthalate dimethyl ester as a raw material to obtain the following product:
[0061]
[0062] The characterization information is as follows:
[0063] Pale yellow solid; 3.6g, yield 82%;
[0064] 1H NMR (400MHz, Methanol-d4) δ7.65(d, J=8.4Hz, 1H), 6.71(d, J=2.8Hz, 1H), 3.85(s, 3H), 3.78(s, 3H).
[0065] 13C NMR (101MHz, Methanol-d4) δ170.80, 167.15, 152.31, 136.68, 131.67, 115.55, 114.17, 112.35, 51.99, 51.30.
[0066] On the basis of the obtained compound, through the step 2 reaction, the following product is obtained:
[0067]
[0068] The characterization information is as follows:
[0069] Pale yellow solid; 1.3g, yield 81%;
[0070] 1H NMR (400MHz, Methanol-d4) δ7.08 (d, J=8.0Hz, 1H), 6.79 (dd, J=6.6, 2.5Hz, 1H), 6.62 (dd, J=8.1, 2.5Hz, 1H) ,4.62(s,2H),4.55(s,2H).
[0071]...
Embodiment 2
[0089] The preparation of embodiment 2 formula 3 compounds
[0090] In this embodiment, step 3 of the above method is adopted to carry out 18 oxygen exchange reaction with leucine as a raw material to obtain the following products:
[0091]
[0092] On the basis of the obtained compound, through the step 4 reaction, the following product is obtained:
[0093]
[0094] On the basis of the obtained compound, through the step 5 reaction, the following product is obtained:
[0095]
[0096] The characterization information is as follows:
[0097] Yellow liquid; 100mg, yield 49.7%.
[0098] On the basis of the obtained compound, react through step 6 to obtain the compound of formula 3:
[0099]
[0100] The characterization information is as follows:
[0101] Yellow liquid; 17.2mg, yield 20%.
[0102] 1H NMR (400MHz, Acetone-d6) δ11.50(s, 1H), 10.60(s, 1H), 10.45(s, 1H), 8.42(d, J=2.1Hz, 1H), 8.15(dt, J= 8.4,1.6Hz,1H),8.08(d,J=8.2Hz,1H),4.41(d,J=10.5Hz,1H),3.07(s,...
Embodiment 5
[0138] Application of Example 5 Protein Quantitative Labeling Reagent in BSA Protein Relative Quantitative Detection
[0139] Utilize the quantitative labeling reagent shown in formula 9 and formula 11 to carry out quantitative accuracy test on BSA protein, add the 100mM TEAB buffer solution of 100ul to the 2ug / ul BSA protein stock solution of 100ul, then add 2ul 1M tris(2 -Carboxyethyl)phosphine (TCEP) solution, react in a metal bath at 65°C for 1 hour, add 7.5ul of 500mM IAA solution after returning to room temperature, and react with shaking at room temperature for 30 minutes. Then add 1ml of acetone, place it at -20°C for 4 hours, then centrifuge at 8000g, 4°C for 10 minutes, suck out the supernatant, redissolve with 1ml 100mM TEAB buffer, add 20ul 0.5ug / ul trypsin solution, and keep at 37°C The reaction was carried out for 16 hours.
[0140] The concentration of peptides was determined by Pierce Quantitative Colorimetric Peptide Assay of Thermo scientific, and the concen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com