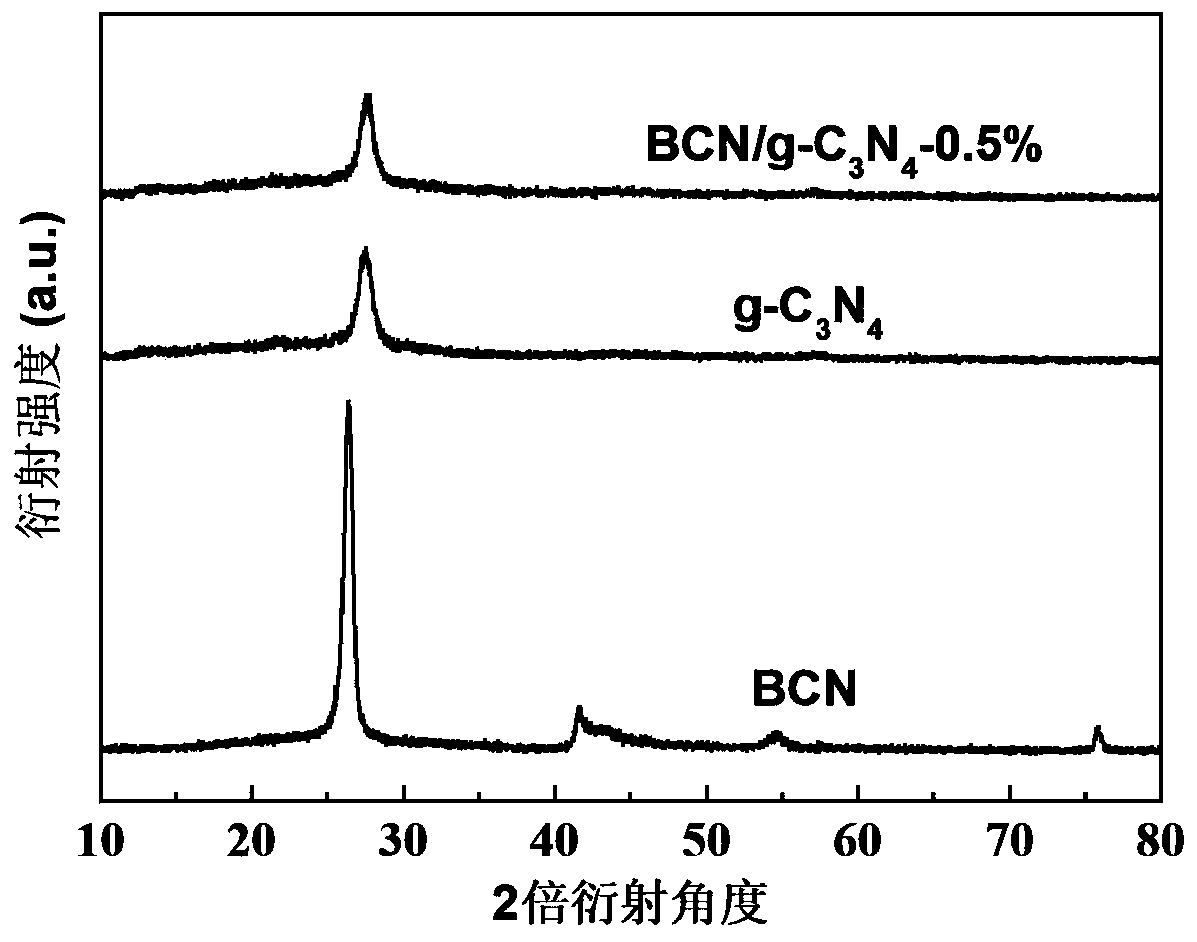
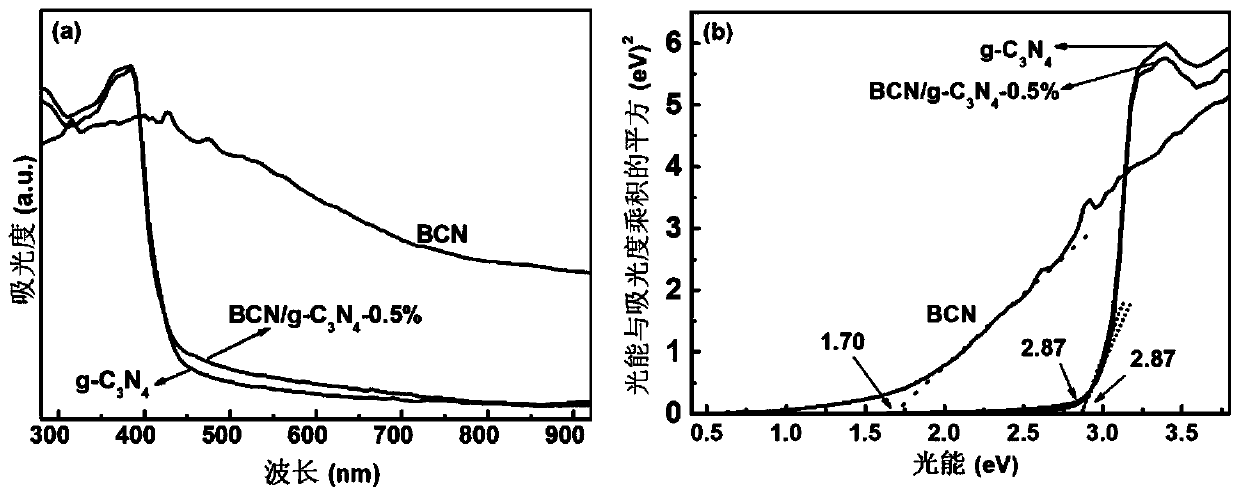
Nonmetal BCN-gC3N4 Van der Waals heterojunction photocatalyst, and preparation method and application thereof
A photocatalyst, g-c3n4 technology, applied in physical/chemical process catalysts, non-metallic elements, chemical instruments and methods, etc., can solve the problems of difficult to widely use carbon nitride, high modification cost, complex process, etc. Facilitate large-scale production, simple method, and the effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Preparation of BCN / g-C 3 N 4 -0.1% van der Waals heterojunction photocatalyst and its photocatalytic hydrogen production
[0033] (1) Preparation of g-C 3 N 4
[0034] Weigh 100g of urea, put it in a drying oven, bake it at 80°C for 12h, take it out, grind it to powder, put it into a crucible, cover it, and put it into a muffle furnace for calcination at 550°C for 4h with a heating rate of 2.5°C / min. After natural cooling, the calcined product was taken out and put into 300mL of 1.5M nitric acid solution, and stirred for 12h. Then carry out washing and suction filtration, wash with deionized water until the pH value of the filtrate is consistent with that of deionized water, and finally put it into a drying oven for drying at 80°C.
[0035] (2) Preparation of BCN nanosheets
[0036] Weigh 2g of boron oxide, 4g of urea, and 0.6g of glucose, put them into a quartz mortar, grind them finely, mix well, then put the mixture into a porcelain boat, cover it, p...
Embodiment 2
[0041] Example 2: BCN / g-C 3 N 4 Preparation of -0.3% Van der Waals Heterojunction Photocatalyst and Photocatalytic Hydrogen Production
[0042] Steps (1)-(2) are the same as in Example 2.
[0043] (3) Preparation of BCN / g-C 3 N 4 -0.3% van der Waals heterojunction photocatalyst
[0044] Weigh 1 g of g-C prepared in step (1) 3 N 4 With 0.003g of BCN nanosheets, ground in a quartz mortar, transferred to a porcelain boat, put the porcelain boat into a tube furnace, and heated in N 2 Heated to 500°C at a heating rate of 5°C / min under air protection, and calcined for 4 hours to obtain BCN / g-C 3 N 4 -0.3% photocatalyst.
[0045] (4) BCN / g-C 3 N 4 -0.3% van der Waals heterojunction photocatalyst for photocatalytic hydrogen production
[0046] The photocatalytic reaction was carried out in a closed reaction system with a total volume of about 250mL, and 50mg BCN / g-C 3 N 4 -0.3% catalyst was uniformly dispersed in 100mL 20vol% pH 11.4 TEOA aqueous solution, then 3% H 2 P...
Embodiment 3
[0047] Example 3: BCN / g-C 3 N 4 Preparation of -0.5% van der Waals heterojunction photocatalyst and photocatalytic hydrogen production
[0048] Steps (1)-(2) are the same as in Example 2.
[0049] (3) Preparation of BCN / g-C 3 N 4 -0.5% van der Waals heterojunction photocatalyst
[0050] Weigh 1 g of g-C prepared in step (1) 3 N 4 With 0.005g of BCN nanosheets, ground in a quartz mortar, transferred to a porcelain boat, put the porcelain boat into a tube furnace, and heated in N 2 Heated to 500°C at a heating rate of 5°C / min under air protection, and calcined for 4 hours to obtain BCN / g-C 3 N 4 -0.5% photocatalyst.
[0051] (4) BCN / g-C 3 N 4 -0.5% van der Waals heterojunction photocatalyst for photocatalytic hydrogen production
[0052] The photocatalytic reaction was carried out in a closed reaction system with a total volume of about 250mL, and 50mgBCN / g-C 3 N 4 -0.5% catalyst was uniformly dispersed in 100mL 20vol% pH 11.4 TEOA aqueous solution, then 3% H 2 Pt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com