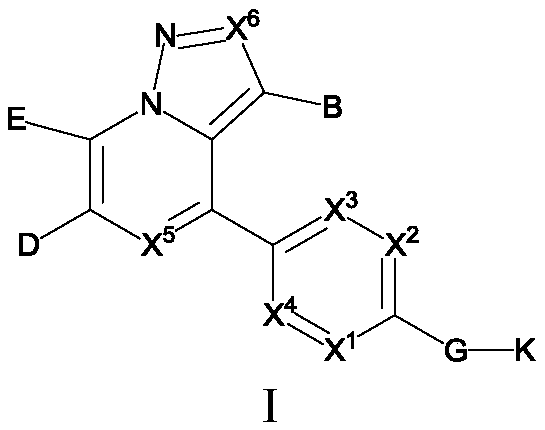
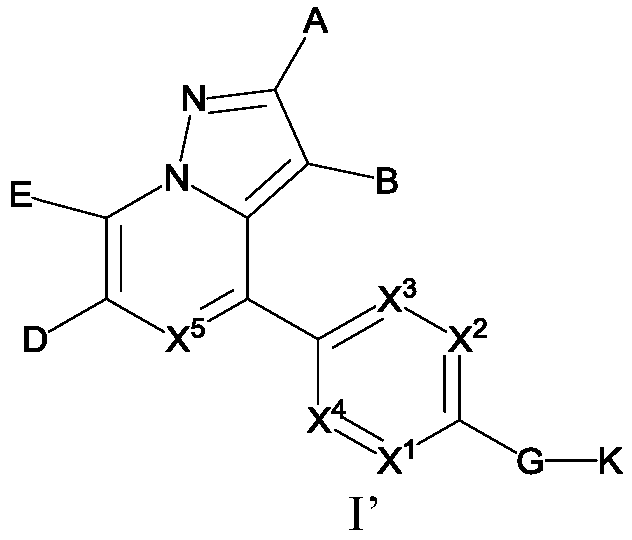
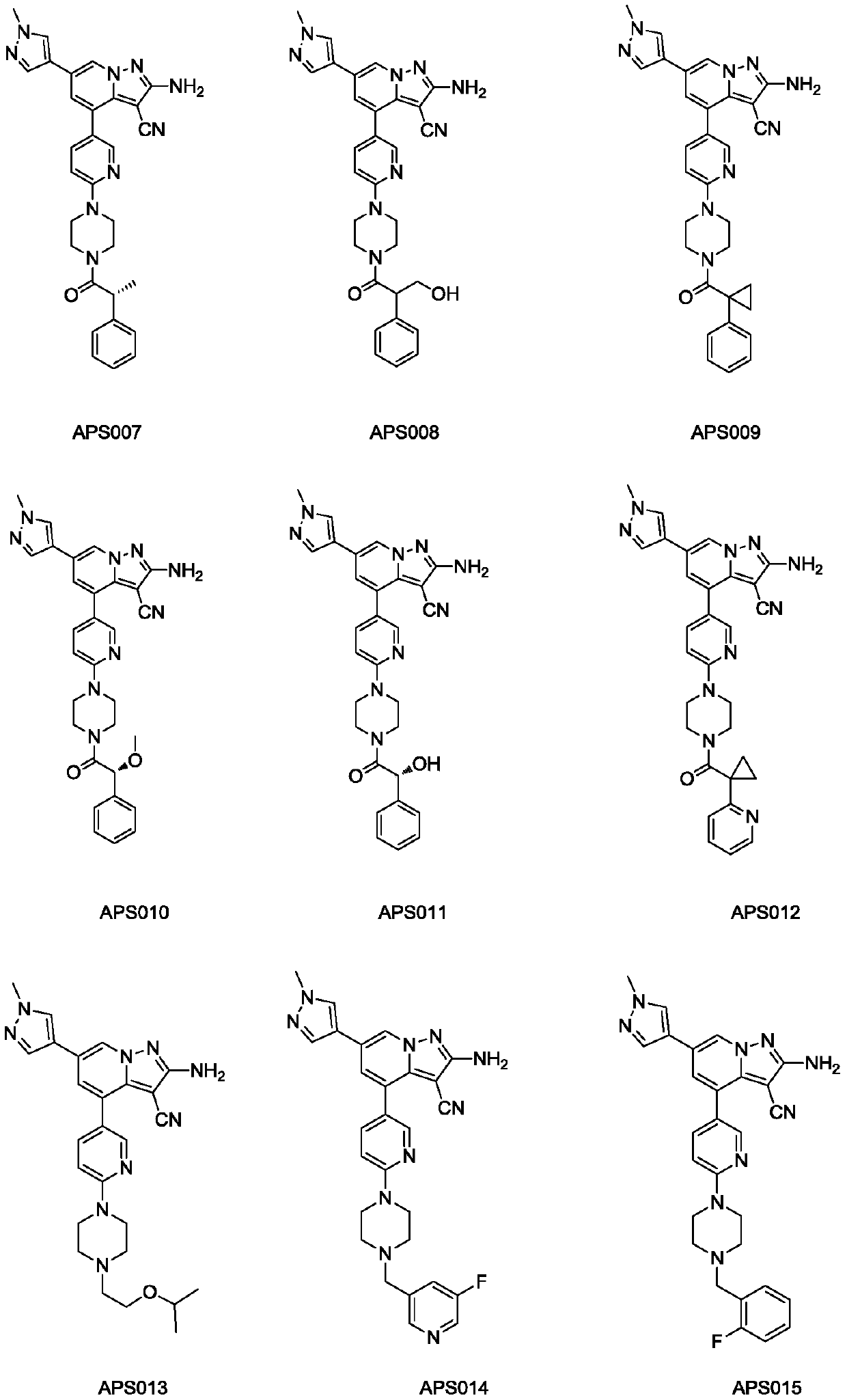
Substituted pyrazole fused ring derivative as well as preparation method and application thereof
A compound and heterocyclic group technology, applied in the field of substituted pyrazole fused ring derivatives and its preparation, can solve problems such as off-target toxicity, poor ORR, and limited treatment options for patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0186] 2-amino-4-(6-(4-(2-(5-fluoropyridin-2-yl)acetyl)piperazin-1-yl)pyridin-3-yl)-6-(1-methyl- 1H-pyrazol-4-yl)pyrazolo[1,5-a]pyridine-3-carbonitrile (APS069)
[0187]
[0188] Step A: tert-Butyl((methylsulfonyl)oxy)carbamate
[0189]
[0190] Under stirring in an ice bath, add 2,4,6-trimethylbenzenesulfonyl chloride (20.0g, 91.5mmol) and tert-butyl N-hydroxycarbamate (12.2g, 91.5mmol) to methyl tert-butyl ether (500mL) solution, triethylamine (13.0mL, 93.3mmol) was slowly added dropwise through a constant pressure dropping funnel, and the temperature of the reaction system was kept below 5°C during the dropwise addition. Under ice bath, the reaction system was stirred for 4.0 hours, filtered under reduced pressure to remove triethylamine hydrochloride, and rinsed with methyl tert-butyl ether three times, all the filtrate was concentrated under reduced pressure at a water bath temperature of less than 15°C to remove most of the methyl Tert-butyl ether; under ice bath...
Embodiment 2
[0237] 2-amino-6-(1-methyl-1H-pyrazol-4-yl)-4-(6-(4-(pyridin-2-ylmethyl)piperidin-1-yl)pyridine-3- base) pyrazolo[1,5-a]pyridine-3-carbonitrile (APS070)
[0238]
[0239] Step A: 1-tert-butoxycarbonyl-4-(pyridin-2-ylmethyl)piperidine
[0240]
[0241] 1-tert-butoxycarbonyl-4-methylenepiperidine (6.0g, 30.4mmol) and 9-borabicyclo[3,3,2]nonane (60.8mL, 30.4mmol, the concentration of the THF solution formed was 2M) mixture was heated to reflux under nitrogen protection for 3 hours, cooled to room temperature, 2-bromopyridine (5.28g, 33.44mmol), 1,1'-bis(diphenylphosphino)ferrocene di Palladium(II) chloride (666mg, 0.91mmol), potassium carbonate (5.04g, 36.48mmol), DMF (80mL), H 2 O (12mL); the reaction mixture was stirred overnight at 60°C, after the TLC spot plate reaction was completed, water was added, and the pH value of the reaction mixture was adjusted to 11 with 10% aqueous sodium hydroxide solution, extracted with ethyl acetate, the combined organic phases, and wa...
Embodiment 3
[0256] 2-Methyl-4-(6-(4-(2-(5-fluoropyridin-2-yl)acetyl)piperazin-1-yl)pyridin-3-yl)-6-(1-methyl -1H-pyrazol-4-yl)pyrazolo[1,5-a]pyridine-3-carbonitrile (APS071)
[0257]
[0258] Step A: Ethyl 2-methyl-6-bromo-4-methoxypyrazolo[1,5-a]pyridine-3-carboxylate
[0259]
[0260] At room temperature, add 2,4,6-trimethylbenzenesulfonic acid 1-amino-3-bromo-5-methoxypyridin-1-ium (2.79g, 6.91mmol) in DMF (25mL) solution Triethylamine (1.93mL, 13.82mmol) was added; the reaction system was cooled to 0°C, ethyl butynoate (1.62mL, 13.82mmol) was added in batches, raised to room temperature and stirred overnight; the reaction was completed, quenched with water, Extract with ethyl acetate, combine the organic phases, wash with water, concentrate under reduced pressure, and separate by column chromatography to obtain the product 2-methyl-6-bromo-4-methoxypyrazolo[1,5-a]pyridine- Ethyl 3-carboxylate (510 mg, 24% yield).
[0261] 1 HNMR (400MHz, CDCl 3 )δ8.13(d, J=1.2Hz, 1H), 6.58(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com