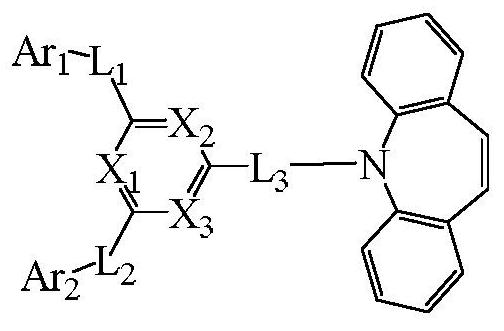
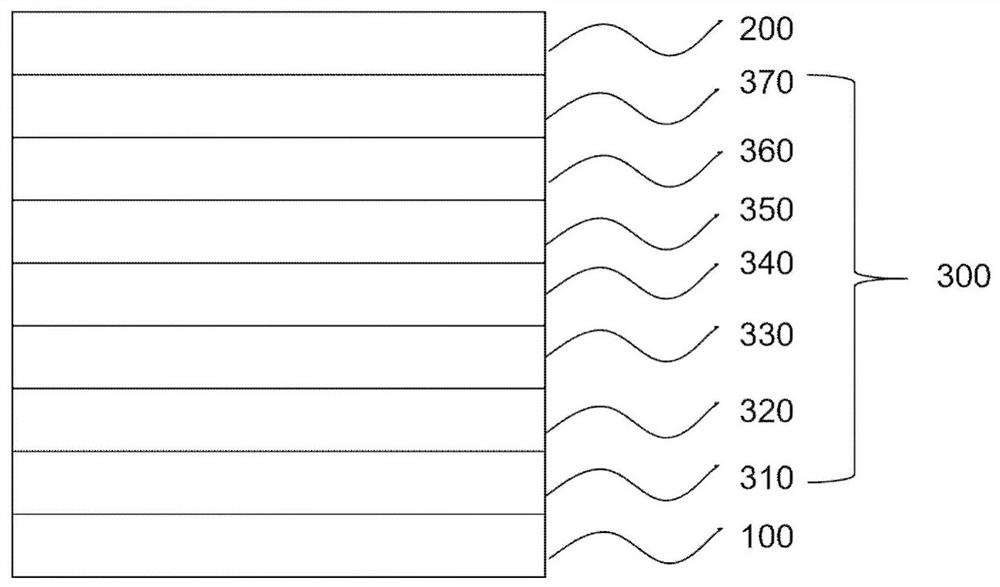
Compound, organic electroluminescent device and display device
A compound and chemical formula technology, applied in the field of organic electroluminescent devices and display devices, can solve the problems of inability to match hole transport materials, weak electron transport ability, high voltage, etc., and achieve favorable electron transport, improve luminous efficiency and External quantum efficiency, the effect of reducing the driving voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0133] Preparation of Synthetic Example 1 Compound 2
[0134]
[0135] Under the protection of nitrogen, intermediate 1 (10.00g, 18.69mmol), 4-biphenylboronic acid (3.70g, 18.69mmol), potassium carbonate (6.46g, 46.72mmol), tetrabutylammonium bromide were successively added to the reaction flask (0.61g, 1.87mmol), toluene 80mL, ethanol 20mL, water 20mL, after the addition, start stirring, heat up to 50 ° C, then add tetrakis (triphenylphosphine) palladium (0.11g, 0.09mmol), after the addition , continue to heat up to 80°C for reflux reaction for 5h, after the reaction is completed, cool down to room temperature, extract with 80mL of toluene, wash the organic phase with 80mL of water*3 times until neutral, dry, filter, and concentrate. Recrystallize with 6 times toluene to LC>99.9%. Drying gave 7.93g of compound 2, yield: 65%. m / z=653.270[M+H] + .
Synthetic example 2
[0136] Preparation of Synthetic Example 2 Compound 13
[0137]
[0138] Under nitrogen protection, intermediate 2 (10.00g, 16.36mmol), 2-pyridineboronic acid (2.01g, 16.36mmol), potassium carbonate (5.65g, 40.91mmol), tetrabutylammonium bromide ( 0.53g, 1.64mmol), toluene 80mL, ethanol 20mL, water 20mL, after the addition, start stirring, heat up to 50°C, then add tetrakis(triphenylphosphine) palladium (0.095g, 0.082mmol), after the addition, Continue to heat up to 80°C and reflux for 3 hours. After the reaction is complete, cool down to room temperature, extract with 80 mL of toluene, wash the organic phase with 80 mL of water*3 times until neutral, dry, filter, and concentrate. Recrystallize with a mixed solvent of 4 times toluene and 2 times n-heptane to LC>99.9%. Drying gave 7.27g of compound 13, yield: 68%. m / z=654.265[M+H] + .
Synthetic example 3
[0139] Preparation of Synthetic Example 3 Compound 19
[0140]
[0141] Under nitrogen protection, intermediate 2 (10.00g, 16.36mmol), 2-naphthylboronic acid (2.81g, 16.36mmol), potassium carbonate (5.65g, 40.91mmol), tetrabutylammonium bromide ( 0.53g, 1.64mmol), toluene 80mL, ethanol 20mL, water 20mL, after the addition, start stirring, heat up to 50°C, then add tetrakis(triphenylphosphine) palladium (0.095g, 0.082mmol), after the addition, Continue to heat up to 80°C and reflux for 2 hours. After the reaction is complete, cool down to room temperature, extract with 80 mL of toluene, wash the organic phase with 80 mL of water*3 times until neutral, dry, filter, and concentrate. Recrystallized with 3 times ethyl acetate to LC>99.9%. Drying gave 6.44 g of compound 19, yield: 56%. m / z=703.286[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com