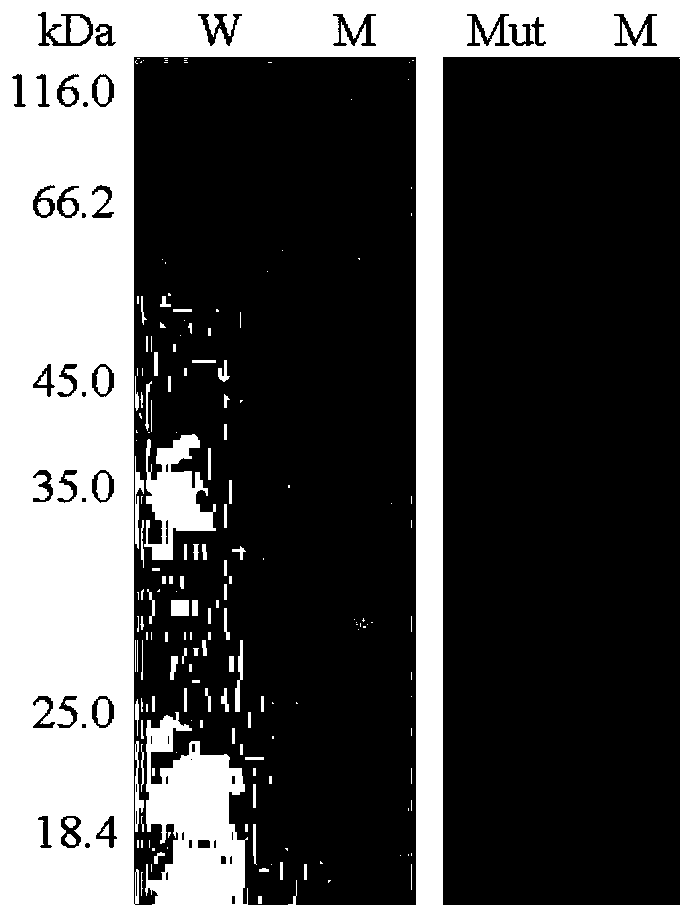
Ammonium sulfate-resistant xylosidase mutant V322DH328DT329E
A technology of V322DH328DT329E and xylosidase, applied in the field of genetic engineering, can solve the problems of simultaneous application, unfavorable degradation of xylo-oligosaccharides, no catalytic activity, etc., and achieves the effect of enhanced stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Construction and transformation of embodiment 1 expression vector
[0033] 1) According to the xylosidase nucleotide sequence KY391885 (SEQ ID NO.4) recorded in GenBank, the coding gene hJ14GH43 of the wild xylosidase HJ14GH43 was synthesized; the coding gene v322d of the mutant enzyme V322D (SEQ ID NO.5) was synthesized ( SEQ ID NO.6) and the coding gene v322dh328dt329e (SEQ ID NO.2) of the mutant enzyme V322DH328DT329E;
[0034] 2) Link the sequences synthesized in (1) with the expression vector pEasy-E1 to obtain the expression vectors containing hJ14GH43, v322d and v322dh328dt329e respectively;
[0035] 3) The ligation products were transformed into Escherichia coli BL21(DE3) to obtain recombinant strains expressing wild enzyme HJ14GH43, mutant enzymes V322D and V322DH328DT329E respectively.
Embodiment 2
[0036] Example 2 Preparation of wild enzyme HJ14GH43 and mutant enzymes V322D and V322DH328DT329E
[0037] The recombinant strains containing hJ14GH43, v322d and v322dh328dt329e were inoculated in LB (containing 100 μg mL -1 Amp) medium, shake rapidly at 37°C for 16h.
[0038] Then inoculate the activated bacterial solution into fresh LB (containing 100 μg mL -1 Amp) culture medium, rapid shaking culture for about 2 ~ 3h (OD 600 After reaching 0.6-1.0), add IPTG at a final concentration of 0.1 mM for induction, and continue shaking culture at 20° C. for about 20 h. Centrifuge at 12000rpm for 5min to collect the bacteria. After suspending the cells with an appropriate amount of pH7.0 Tris-HCl buffer solution, the cells were ultrasonically disrupted in a low-temperature water bath. After the crude enzyme solution concentrated in the cells was centrifuged at 12,000 rpm for 10 min, the supernatant was aspirated and the target protein was affinity-eluted with Nickel-NTAAgarose an...
Embodiment 3
[0040] Example 3 Determination of the properties of the purified wild enzyme HJ14GH43 and mutant enzymes V322D and V322DH328DT329E
[0041] The activities of the purified wild enzyme HJ14GH43 and mutant enzymes V322D and V322DH328DT329E were measured by the pNP method: pNPX was dissolved in buffer to make the final concentration 2mM; the reaction system contained 50μL of appropriate enzyme solution and 450μL of 2mM substrate; After preheating at the reaction temperature for 5 minutes, add the enzyme solution and react for an appropriate time, then add 2mL of 1M Na 2 CO 3 The reaction was terminated, and the released pNP was measured at a wavelength of 405 nm after cooling to room temperature; 1 enzyme activity unit (U) was defined as the amount of enzyme required to decompose the substrate to produce 1 μmol pNP per minute.
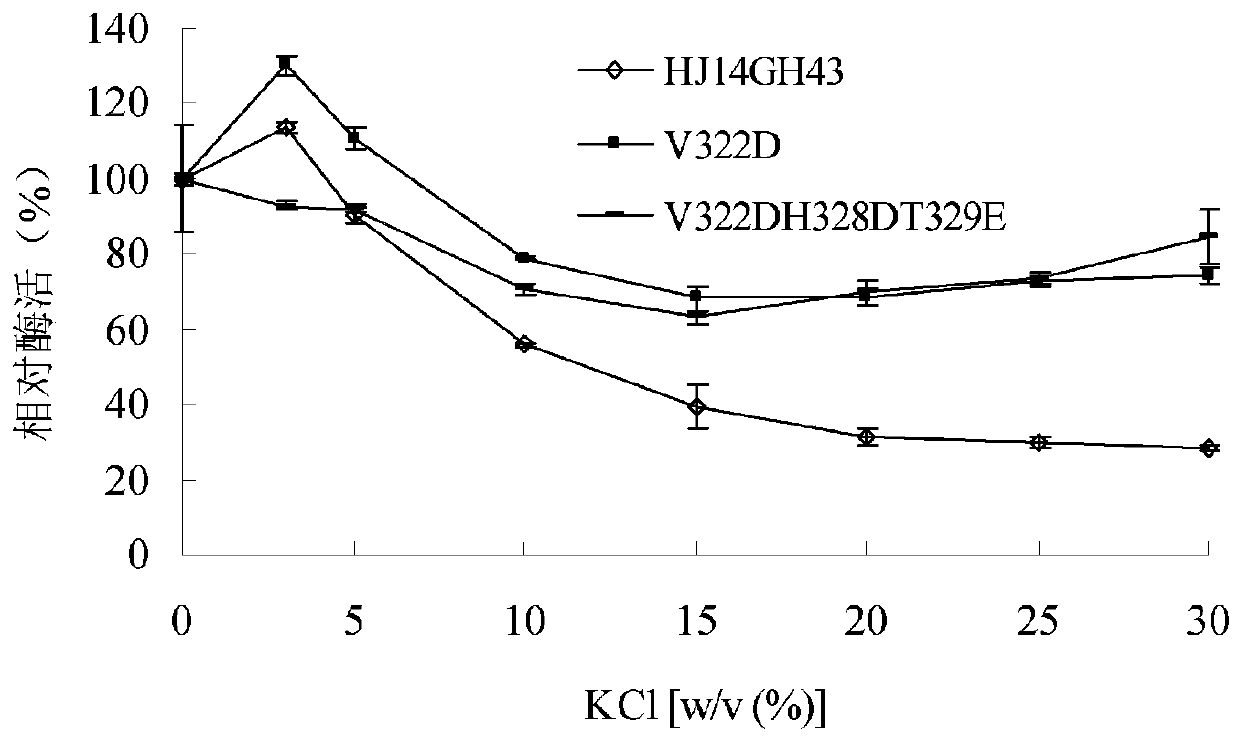
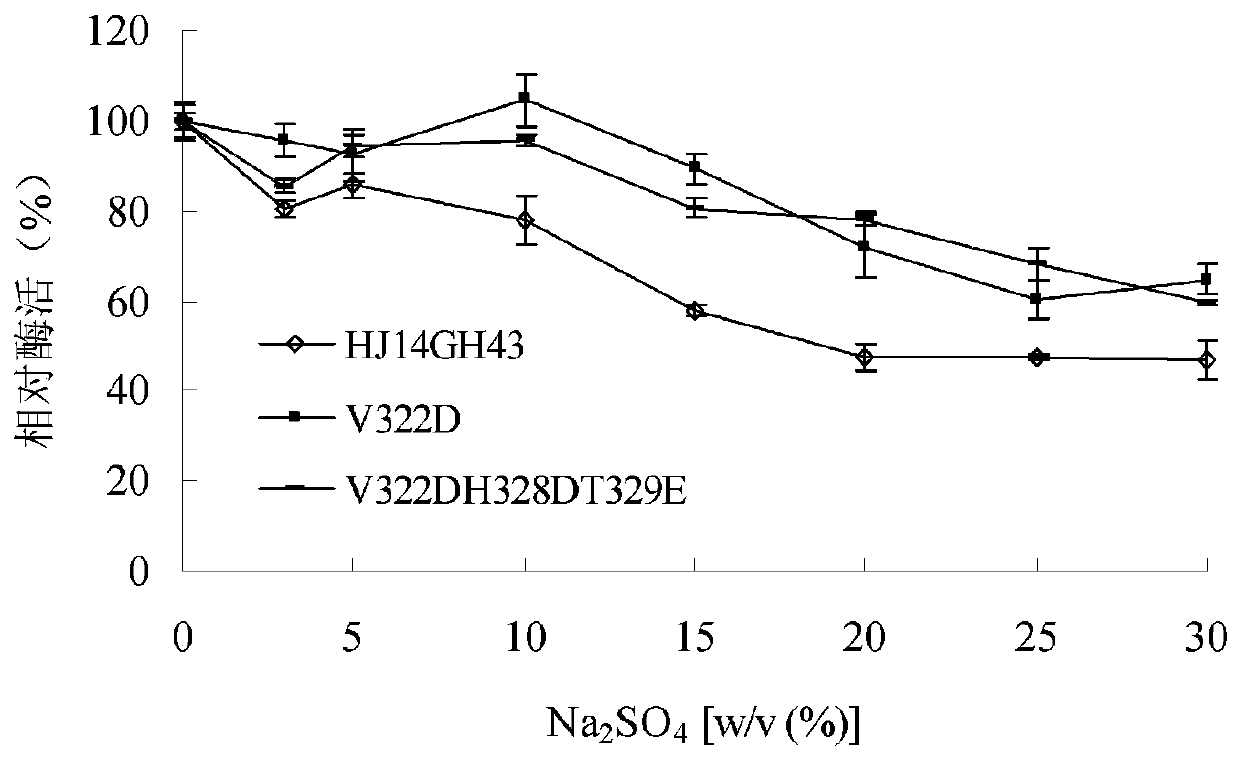
[0042] 1) Stability of purified wild enzyme HJ14GH43 and mutant enzymes V322D and V322DH328DT329E in KCl
[0043] The purified enzyme solution was place...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com