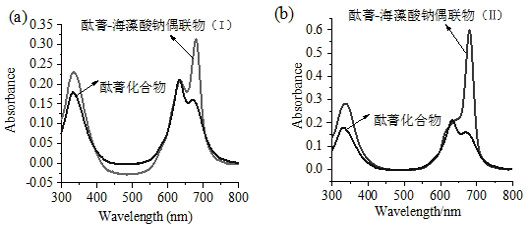
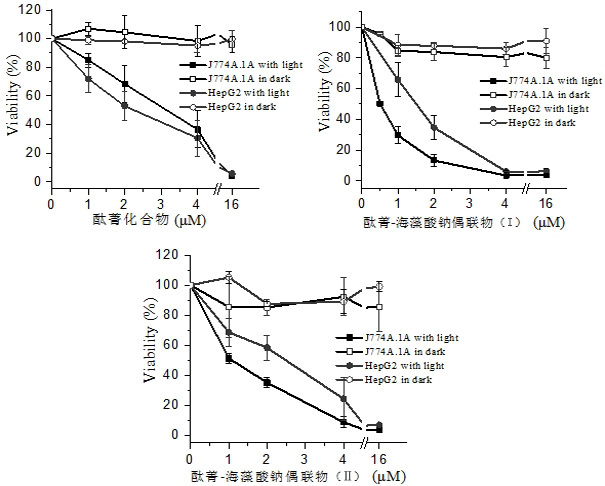
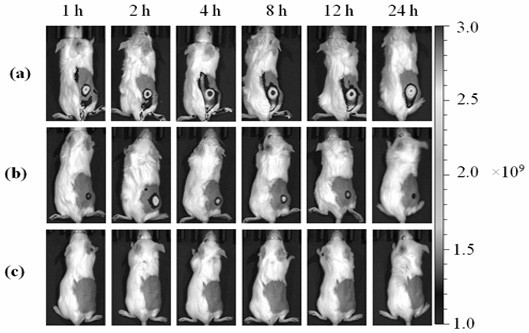
Application of Sodium Alginate as Targeting Carrier of Antineoplastic Drugs
An anti-tumor drug, sodium alginate technology, applied in anti-tumor drugs, anti-bacterial drugs, drug combinations, etc., can solve problems such as poor biological selectivity and easy aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] A conjugate prepared using sodium alginate as a targeting carrier, the preparation method comprising the following steps:
[0031] 1) Disperse sodium alginate in 70 vol% formic acid / water solution or 1M hydrochloric acid / ethanol solution, stir in ice water for 1 hour, transfer to 20~35°C and continue stirring for 5 hours, filter, and obtain crude alginic acid Wash with 70vol%~95vol% ethanol aqueous solution and acetone, disperse it in purified water after vacuum drying, slowly add 10~40 wt% tetrabutylammonium hydroxide aqueous solution to adjust pH=8±1, then freeze immediately, and carry out lyophilization, Obtain tetrabutylammonium alginate;
[0032] 2) Dissolve the obtained tetrabutylammonium alginate in DMSO, and stir at 20-35°C under the protection of nitrogen to dissolve completely; then add CMPI dissolved in DMSO and activate for 1 hour; then add phthalocyanine compound and three Ethylamine, continue to react for 20 hours, finally add 2.5M NaCl solution to the re...
Embodiment 1
[0042] (1) Preparation of alginic acid
[0043] Disperse 2 g (9.1 mmol) of low-molecular-weight sodium alginate (MW=40kDa, 9.1 mmol of repeating units) in 100 mL of formic acid / water solution (7:3, v / v) at 4°C, and stir in ice water for 1 h. Transfer to 20~35°C and continue to stir for 5 hours, then filter, the obtained crude alginic acid is washed with 300mL of absolute ethanol, and then washed with 100mL of acetone, and vacuum-dried at 40°C to obtain 1.44g of light yellow powdery solid, which is alginic acid (1).
[0044] Product Characterization Data: FTIR (ATR): υ (OH) 3371 cm -1 ; υ as (CH) 2925cm -1 ; υ (C=O inCOOH) 1723 cm -1 ; υ as (COO − ) 1636 cm -1 ; υ s (COO - ) 1402 cm -1 ; υ (C-O-C) 1232 cm -1 ; υ (C-OH)1029 cm -1 .
[0045] (2) Synthesis of tetrabutylammonium alginate
[0046] Disperse 1g (repeating unit 5mmol) of alginic acid in 200mL of purified water, then slowly adjust the pH=8±1 with 40wt% tetrabutylammonium hydroxide aqueous sol...
Embodiment 2
[0049] Adjust the molecular weight of sodium alginate in step (1) of Example 1 to 1230 kDa, except for washing with 300mL ethanol / water solution (7:3, V:V) and then washing with 100mL acetone, other steps are carried out in the same way In Example 1, 1.51g of alginic acid (2) and 2.03g of tetrabutylammonium alginate (2) were obtained.
[0050] Product characterization data are as follows:
[0051] Alginic acid (2): FTIR (ATR): υ (OH) 3359 cm -1 ; υ as (CH) 2925cm -1 ; υ (C=O in COOH)1721 cm -1 ; υ as (COO − ) 1635 cm -1 ; υ s (COO - ) 1394 cm -1 ; υ (C-O-C) 1236cm -1 ; υ (C-OH) 1028cm -1 .
[0052] Tetrabutylammonium Alginate (2): FTIR (ATR): υ (OH) 3384 cm -1 ; υ (CH) 2961cm -1 , 2935 cm −1 and 2875cm −1 ; υ as (COO - ) 1603cm -1 ; δ as (CH 2 and CH 3 ) 1487 cm −1 and 1465 cm −1 ; δ s (CH 3 )1385 cm −1 ;(C-O) 1315 cm −1 and 1285cm −1 ;(C-O-C) 1171 cm −1 , 1147 cm −1 and 1099cm −1 , (C-OH) 1031 cm −1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface potential | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com