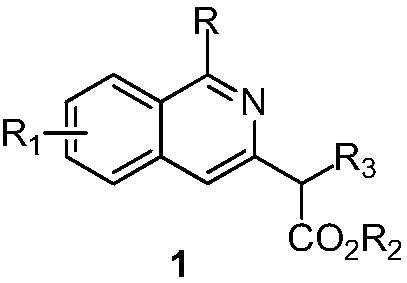
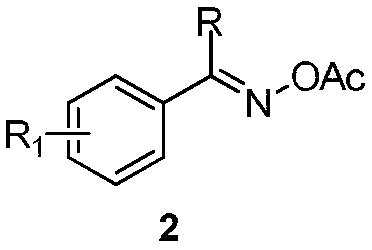
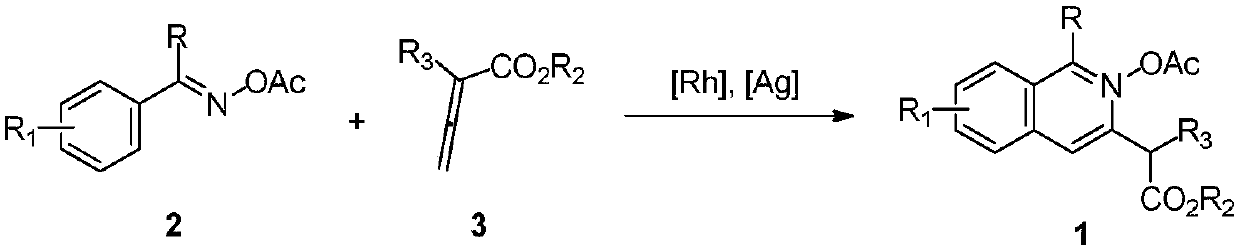
2-(3-isoquinolinyl)-ethyl propionate derivative and synthesis method thereof
A technology based on ethyl propionate and isoquinoline, applied in the field of 2--ethyl propionate derivatives and its synthesis, can solve the problems of no reports, etc., and achieve the effects of simple operation, mild reaction conditions, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
[0029] The specific process is: weigh O-acetyl oxime 2a (53mg, 0.3mmol) in the glove box, [RhCp*Cl 2 ] 2 (3.7mg, 0.006mmol), pivalic acid (6.1mg, 0.06mmol), was added to a 25mL tube with a branch port, and ethyl allenoate 3a (76mg, 0.6mmol) was added under nitrogen atmosphere, 1, 2-Dichloroethane (3 mL) was reacted at 60° C. for 24 h. After the reaction was complete, the solvent was removed by rotary evaporation under reduced pressure, and then silica gel column chromatography (petroleum ether (60-90° C.) / ethyl acetate: 20:1, v / v) was used to obtain a colorless liquid product 1a (62 mg, harvested rate 85%). The target product was confirmed by NMR spectroscopy.
Embodiment 2
[0031]
[0032] The reaction steps and operations are the same as in Example 1, except that the O-acetyl oxime added to the reaction system is 2b (57 mg, 0.3 mmol). The reaction was stopped, and the target product 1b (68 mg, yield 88%) was obtained as a light yellow liquid after post-processing. The target product was confirmed by NMR and high-resolution mass spectrometry.
Embodiment 3
[0034]
[0035] The reaction steps and operations are the same as in Example 1, except that the O-acetyl oxime added to the reaction system is 2c (62 mg, 0.3 mmol). The reaction was stopped, and the target product 1c (75 mg, yield 92%) was obtained as a light yellow liquid after post-processing. The target product was confirmed by NMR and high-resolution mass spectrometry.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com