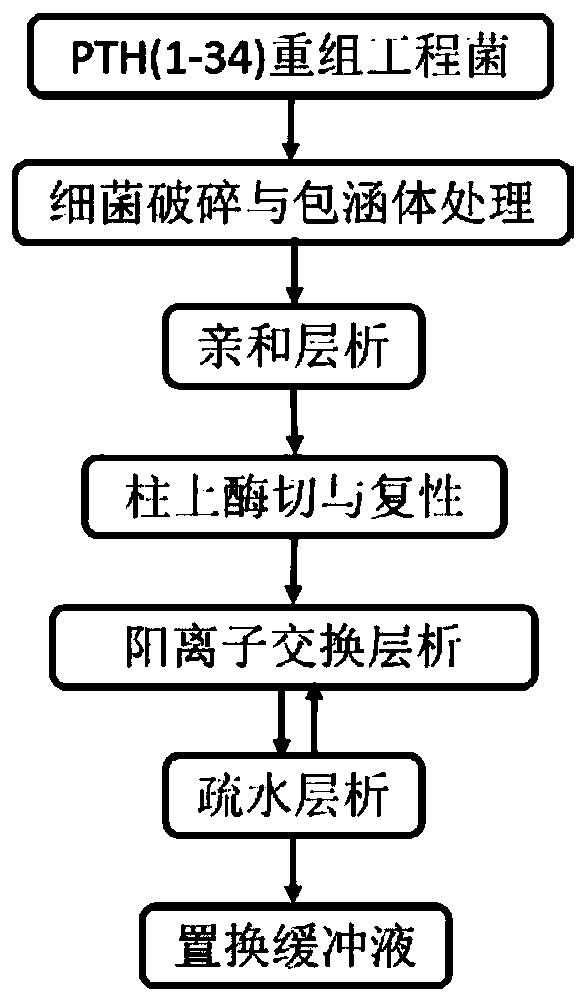
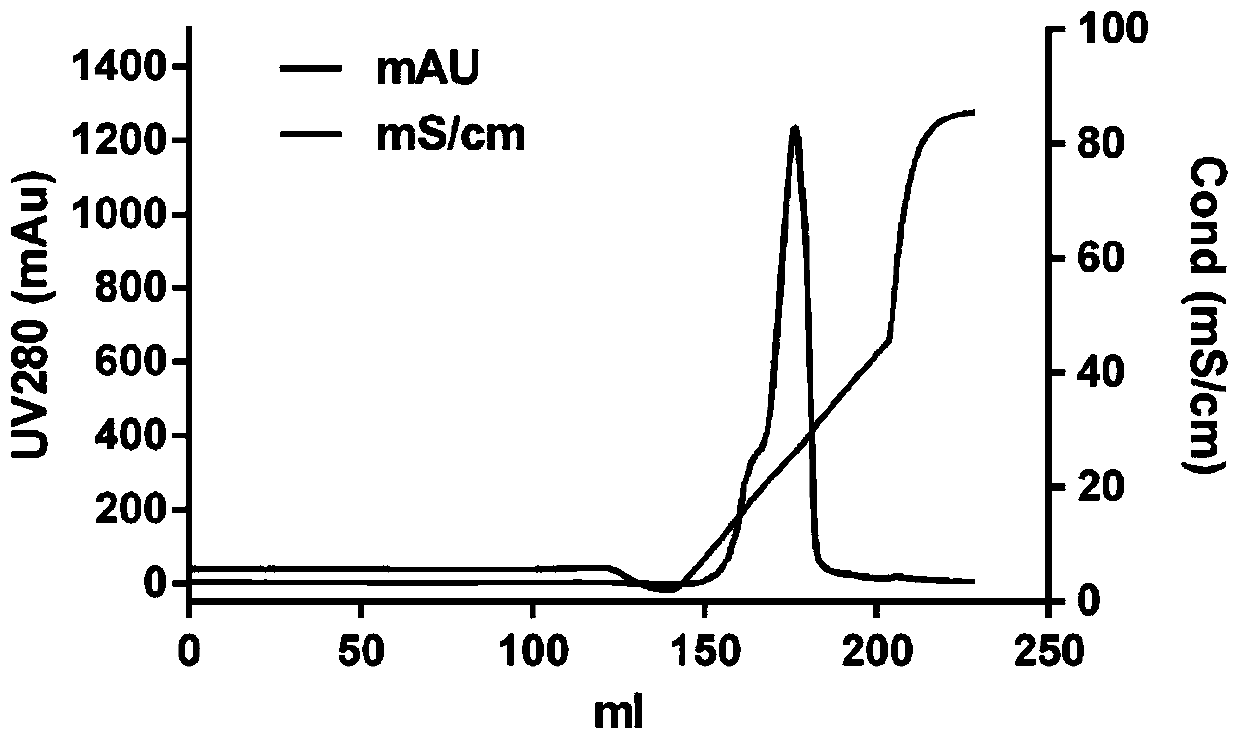
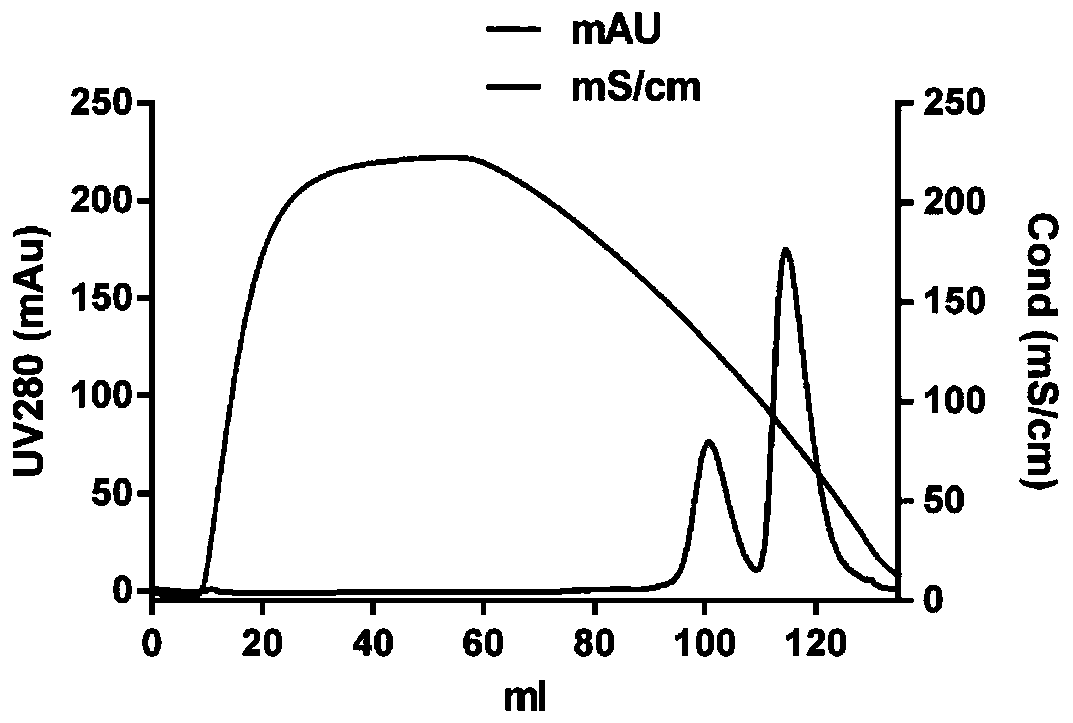
Purification method of recombinant parathyroid hormone PTH (1-34)
A parathyroid hormone, separation and purification technology, applied in the field of separation and purification of recombinant parathyroid hormone PTH, can solve the problems of complex process flow, influence on polypeptide activity, low yield, etc., and achieve the effect of shortening purification process and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Construction and expression of embodiment 1 recombinant PTH (1-34) engineering bacteria
[0057] PTH(1-34) is the amino acid sequence of SEQ ID NO:1, and the encoding DNA code is optimized to SEQ ID NO:2.
[0058] Three segments of genes including TEV enzyme cleavage site (ENLYFQ), enterokinase enzyme site (DDDDK) and factor Xa cleavage site (IEGR) were designed at the N-terminus of PTH (1-34). Add (GGGGS)3, His6 and SSGSSG sequentially from right to left, add BamHI site GGATCC at the 5' end of each gene, add stop codon TGATAA, HindIII site AAGCTT, NotI site GCGGCCGC at the 3' end, and submit the sequence to Shanghai Synthesized by Bioengineering Ltd.
[0059] Respectively use BamHI+HindIII and BamHI+NotI to double-enzyme digest the synthesized three-segment gene and each vector as shown in Table 1, and recover the target fragment and vector respectively. The construction of the recombinant expression vector was carried out according to the manner in Table 1.
[0060...
Embodiment 2
[0064] Embodiment 2: the optimized construction of recombinant PTH (1-34) engineering bacterium
[0065] The preliminary results obtained by summarizing the fusion protein expressed by the recombinant vector constructed in Example 1 are shown in Table 2,
[0066] Table 2 The expression of each recombinant engineered bacteria
[0067]
[0068] If it is a soluble expression fusion protein, the obtained target protein PTH(1-34) is an oxidized form; if it is an inclusion body expression, the fusion protein needs to be fully refolded before protease digestion, and the existing purpose must be followed. In the old way of protein preparation, a reverse-phase purification step must be added to the purification process, and a large amount of organic solvents are used in the preparation process.
[0069] In order to overcome the disadvantages of the prior art, the optimal construction strategy of the present application for the recombinant PTH (1-34) engineering bacteria is: the rec...
Embodiment 3
[0071] Example 3 Bacteria Breakdown and Treatment of Inclusion Body
[0072] Bacteria resuspension: the pET28a-PTH(1-34) / BL21 Escherichia coli engineered bacteria were fermented at high density, and the bacteria were collected by centrifugation for later use. Take about 50g of bacteria, mix and suspend with PBS buffer solution (liquid A) of pH6.0-pH8.5 according to the weight (g): volume (ml) ratio of 1:5-10, and pre-cool at 4°C.
[0073] High-pressure sterilization: Use distilled water to flush the pipeline of the high-pressure homogenizer, and turn on the low-temperature circulation system to pre-cool to 1-4°C for later use. Add the pre-cooled suspended bacteria liquid to a high-pressure homogenizer, maintain the pressure at 60-80Mpa to break the bacteria 3-5 times, take a smear of the broken bacteria liquid and stain it with crystal violet, and the unbroken bacteria in each field of view of the oil immersion lens is less than 1-2. For the destruction of bacteria completely...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com