Preparation method and application of natural walnut shell powder sulfonic acid catalyst
A technology of walnut shell and powder sulfonic acid is applied in the field of preparation of natural walnut shell powder sulfonic acid catalyst, can solve the problems of low product yield, high cost, ungreen reaction conditions, etc., and achieves high reaction activity, short reaction time, Effects that are easy to prepare
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
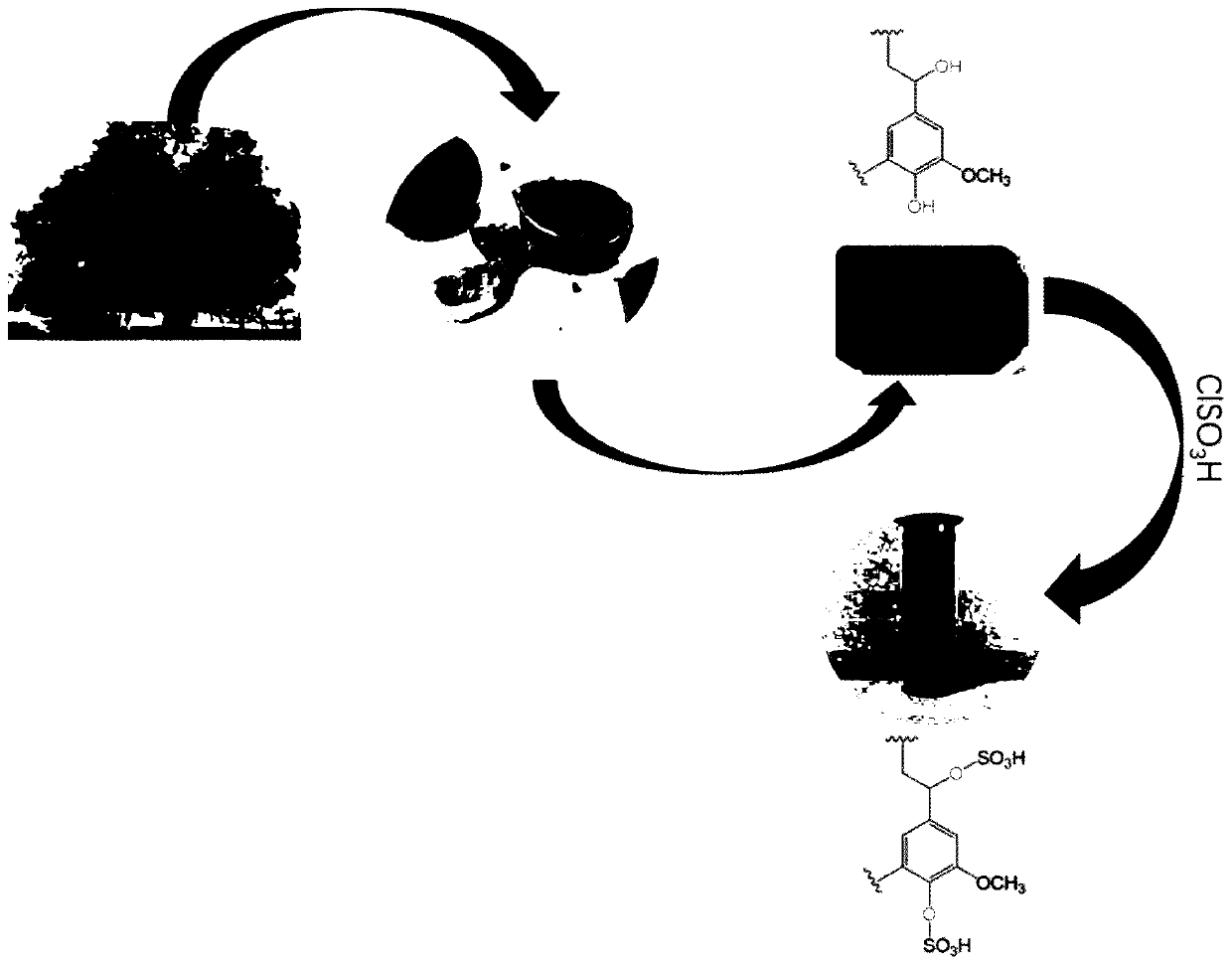
[0026] like figure 1 Shown, the natural walnut shell powder sulfonic acid catalyst WSP-SO in the following embodiment 3 The preparation method of H comprises:
[0027] (1) Grind 10g of natural walnut shells into powder, add CH 2 Cl 2 30mL and stir well;
[0028] (2) Add 4 mL of chlorosulfonic acid dropwise to the material obtained in step (1) at 0° C., stir at room temperature for 5 h after the addition is complete, then fully wash with ethanol, and finally dry at room temperature to obtain the Natural walnut shell powder sulfonic acid catalyst WSP-SO 3 H.
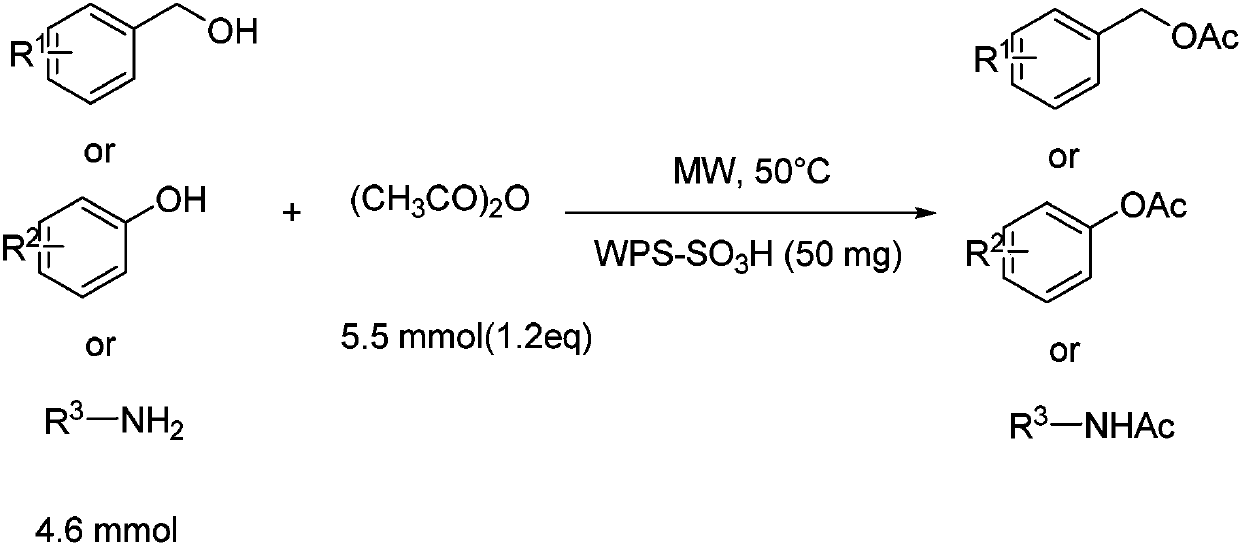
[0029] The synthetic route of following each embodiment is as follows:
[0030]
[0031] Among them, R 1 is hydrogen, 2-chloro or 4-chloro;
[0032] R 2 is 2-chloro, 4-bromo, 4-nitro or naphthyl;
[0033] R 3 is hydrogen, 2-chloro, 2-nitro, benzyl, 4-methoxy or 4-methyl.
Embodiment 1
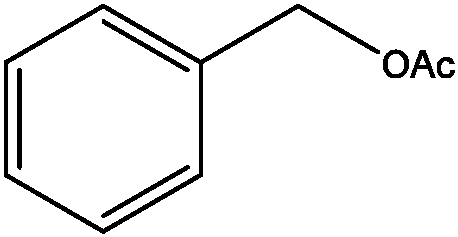
[0035] The preparation of benzyl acetate (structural formula is as follows):
[0036]
[0037] Benzyl alcohol (4.6mmol), acetic anhydride (1.2eq), WSP-SO 3 H (50mg), magnetons, then put the test tube into the microwave reactor, set the reaction conditions as follows: microwave radiation power 300W, reaction temperature 50°C, reaction time is 5min, the reaction process is monitored by TLC, after the reaction is completed, the product is collected and passed The product was separated and purified by centrifugation, rotary evaporation and column chromatography with a yield of 95%.
[0038] The infrared and nuclear magnetic characterization of this product are as follows: FT-IR (KBr, cm -1 ): 3034, 2955, 1734, 1497, 1455, 1379, 1024, 964, 920; 1 H NMR (500MHz, CDCl 3 )δ: 7.41-7.34 (m, 5H), 5.15 (s, 2H), 2.13 (s, 1H).
Embodiment 2
[0040] The preparation of 2-chlorobenzyl acetate (structural formula is as follows):
[0041]
[0042] Add 2-chlorobenzyl alcohol (4.6mmol), acetic anhydride (1.2eq), WSP-SO 3 H (50mg), magnetons, then put the test tube into the microwave reactor, set the reaction conditions as follows: microwave radiation power 300W, reaction temperature 50°C, reaction time is 5min, the reaction process is monitored by TLC, after the reaction is completed, the product is collected and passed The product was obtained by separation and purification by centrifugation, rotary evaporation and column chromatography.
[0043] The infrared and nuclear magnetic characterization of this product are as follows: FT-IR (KBr, cm -1 ): 3065, 2959, 1735, 1478, 1443, 1379, 1028, 969, 919; 1 H NMR (500MHz, CDCl 3 )δ: 7.38(d, 1H), 7.30-7.20(m, 4H), 5.21(s, 1H), 2.10(t, 3H).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap