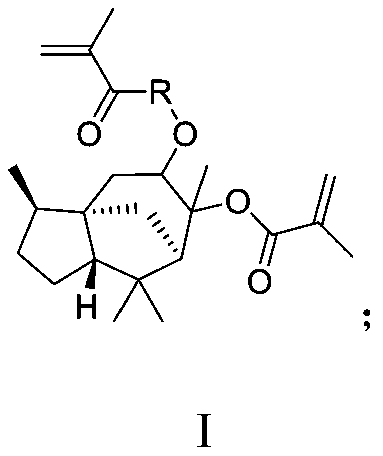
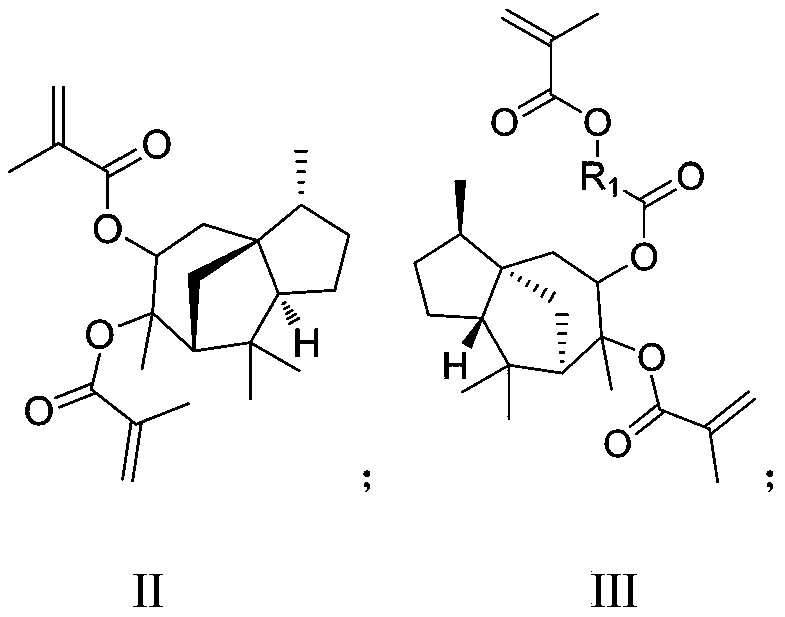
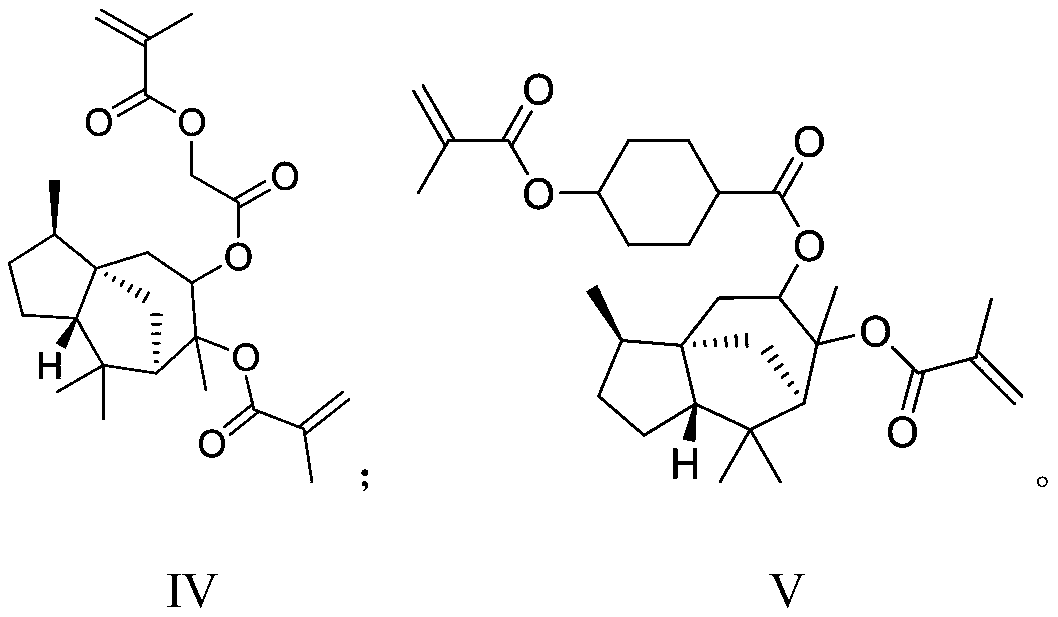
Photoresist resin monomer synthesized from alpha-cedrene and synthesis method thereof
A technology of resin monomer and synthesis method, which is applied in the field of photoresist resin monomer and its synthesis, can solve the problem of low resolution of photolithographic patterns, and achieve the goals of improving resolution, increasing etch resistance, and increasing dissolution rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] This embodiment provides a first intermediate with a structural formula of Formula 1. The reaction route of the synthesis method of the first intermediate is as follows:
[0034]
[0035] It specifically includes the following steps:
[0036] First dissolve α-cedarene (10g, 48.9mmol) in dichloromethane (200g), add 85% mass fraction of m-chloroperoxybenzoic acid (12.9g, 63.7mmol) at 0℃ in an ice water bath Into the above solution, and placed at 25°C and stirred for 8 hours to obtain a reaction solution. Next, filter the reaction solution and use saturated NaHCO sequentially 3 Wash the filtrate with aqueous solution, water and brine, and use anhydrous Na for the organic phase 2 SO 4 After drying and concentration, a solid first intermediate (formula 1, 9 g, 40.8 mmol, molar yield 83.5%) was obtained.
Embodiment 2
[0038] This embodiment provides a photoresist resin monomer synthesized from α-cedarene. The reaction route of the synthesis method of the photoresist resin monomer is as follows:
[0039]
[0040] It specifically includes the following steps:
[0041] Under the protection of nitrogen, the first intermediate (formula 1, 2g, 9.1mmol) provided in Example 1 above was dissolved in dimethylformamide (50g), and after dissolution, 1,8-diazabicyclo[ 5.4.0] Undec-7-ene (0.35 g, 2.3 mmol) and methacrylic anhydride (4.2 g, 27.2 mmol), then stirred at 120°C for 12 hours. The reaction solution was extracted with ether to obtain an extract, and the extract was washed with water and concentrated to obtain a solid photoresist resin monomer (formula 2, 2.2 g, 5.9 mmol, molar yield 64.7%).
Embodiment 3
[0043] This embodiment provides a photoresist resin monomer synthesized from α-cedarene. The reaction route of the synthesis method of the photoresist resin monomer is as follows:
[0044]
[0045] It specifically includes the following steps:
[0046] S1. Dissolve glycolic acid (2g, 26.3mmol) and triethylamine (2.7g, 26.7mmol) in dichloromethane (50g), then add methacrylic acid chloride (2.8g, 26.7mmol) dropwise to the above mixture In the solution, stir for 30 minutes at 0°C under nitrogen protection, and then continue stirring for 4 hours at room temperature. Filter and concentrate the filtrate to obtain a crude product. The crude product is purified by column chromatography to obtain liquid compound 3-1 (Formula 3-1, 3 g, 20.8 mmol, molar yield 79.1%).
[0047] S2, under the protection of nitrogen, the first intermediate (2g, 9.1mmol) provided in the above example was dissolved in dimethylformamide (30g), after dissolution, 1,8-diazabicyclo[5.4. 0] Undec-7-ene (0.35 g, 2.3 mmol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com