Surface-modified lithium-rich layered transition metal oxide as well as preparation method and application thereof
A transition metal and surface modification technology, applied in the direction of active material electrodes, electrochemical generators, electrical components, etc., can solve problems such as difficult to obtain effects, achieve poor intrinsic conductivity, improve cycle stability, and increase conductivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
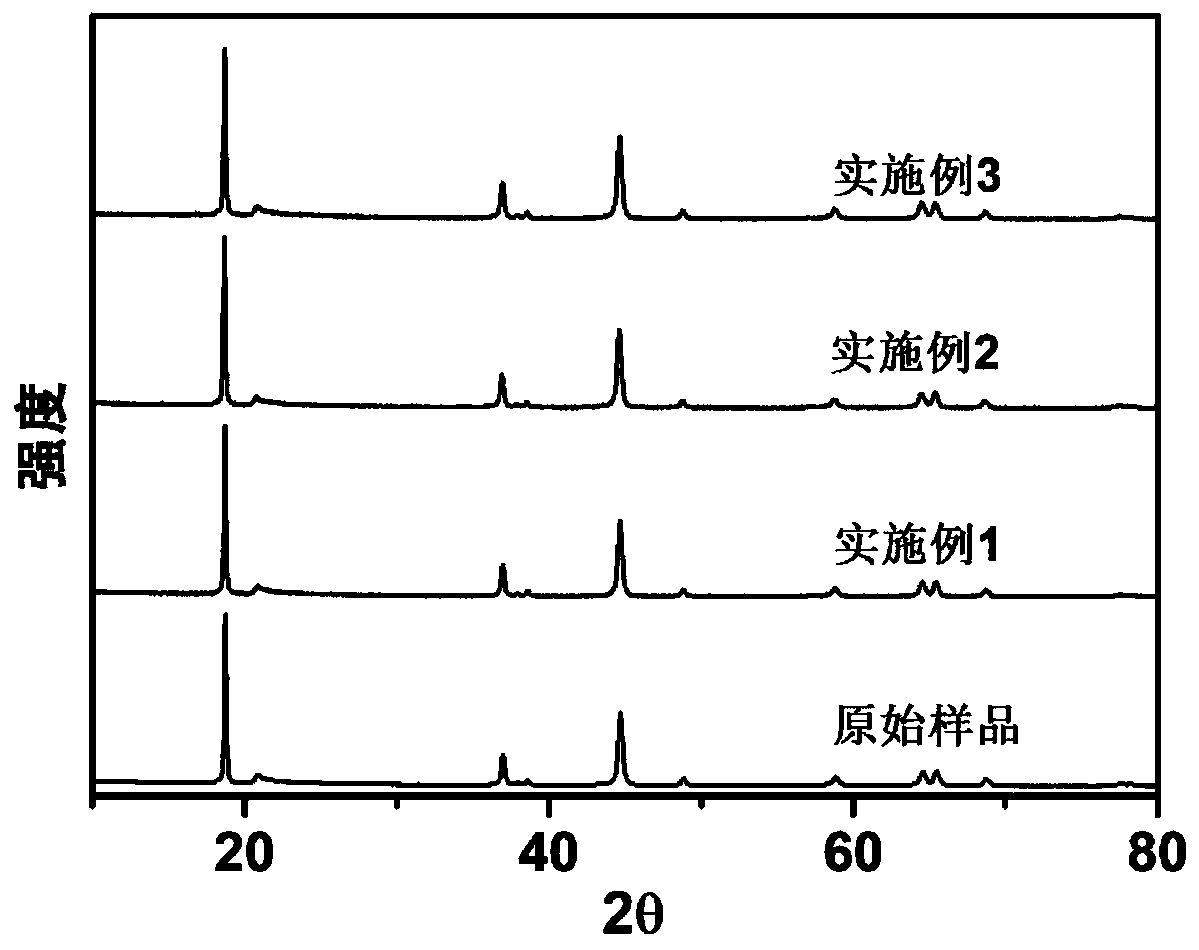
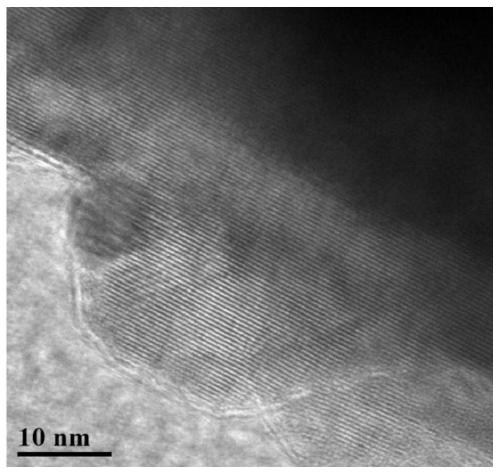
[0033] 1. The 0.008mol transition metal carbonate (Mn 0.75 Ni 0.25 CO 3 ) Or transition metal hydroxide precursor (Mn 0.75 Ni 0.25 (OH) 2 ) And excess lithium carbonate and molten salt sodium chloride and potassium chloride weighed in molar quantities (the molar ratio of precursor to molten salt is 1:5, and the molar ratio of sodium chloride and potassium chloride is 1:1 ) Mix uniformly, place the mixture in a muffle furnace, heat up to 850°C at a rate of 5°C / min and keep it for 12 hours, then cool to room temperature naturally, after washing, filtering, and drying to obtain the original sample that is lithium-rich Layered transition metal oxide (Li 1.2 Mn 0.6 Ni 0.2 O 2 ).
[0034] 2. Mix the lithium-rich layered transition metal oxide and urea with a molar ratio of 5:1 and place them in a polytetrafluoroethylene reaction vessel filled with an inert atmosphere and seal the reaction vessel. Transfer the reaction vessel to a temperature of 150 It was kept in a blast drying oven at...
Embodiment 2
[0037] 1. The 0.008mol transition metal carbonate (Mn 0.75 Ni 0.25 CO 3 ) Or transition metal hydroxide precursor (Mn 0.75 Ni 0.25 (OH) 2 ) And excess lithium carbonate and molten salt sodium chloride and potassium chloride weighed in molar quantities (the molar ratio of precursor to molten salt is 1:5, and the molar ratio of sodium chloride and potassium chloride is 1:1 ) Mix uniformly, place the mixture in a muffle furnace, heat up to 850°C at a rate of 5°C / min and keep it for 12 hours, then cool to room temperature naturally, after washing, filtering, and drying to obtain the original sample that is lithium-rich Layered transition metal oxide (Li 1.2 Mn 0.6 Ni 0.2 O 2 ).
[0038] 2. At a molar ratio of 5:1, mix the lithium-rich layered transition metal oxide and urea uniformly, and place them in a reaction vessel filled with inert atmosphere of polytetrafluoroethylene and seal the reaction vessel. Transfer the reaction vessel to a temperature of 180°C. In a blast drying oven a...
Embodiment 3
[0042] 1. The 0.008mol transition metal carbonate (Mn 0.75 Ni 0.25 CO 3 ) Or transition metal hydroxide precursor (Mn 0.75 Ni 0.25 (OH) 2 ) And excess lithium carbonate and molten salt sodium chloride and potassium chloride weighed in molar quantities (the molar ratio of precursor to molten salt is 1:5, and the molar ratio of sodium chloride and potassium chloride is 1:1 ) Mix uniformly, place the mixture in a muffle furnace, heat up to 850°C at a rate of 5°C / min and keep it for 12 hours, then cool to room temperature naturally, after washing, filtering, and drying to obtain the original sample that is lithium-rich Layered transition metal oxide (Li 1.2 Mn 0.6 Ni 0.2 O 2 ).
[0043] 2. With a molar ratio of 5:1, mix the lithium-rich layered transition metal oxide and urea evenly and place them in a reaction vessel filled with inert atmosphere in a polytetrafluoroethylene and seal the reaction vessel. The reaction vessel is transferred to a temperature of 210°C In a blast drying o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com