Recombinant human type III collagen having functional structure and expression method thereof
A collagen, functional structure technology, applied in the direction of animal/human protein, microbial-based methods, connective tissue peptides, etc., to achieve good biological activity and collagen stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Gene Design and Synthesis
[0042] (1) Genetic design:
[0043] According to the amino acid sequence of human type III collagen (UniProtKB / Swiss-Prot: P02461.4), SEQ ID NO: 1:
[0044] MYDSYDVKSGVAVGGLAGYPGPPGPPPGPAGPPGPPGPPGTSGHPGSPGSPGYQGPPGEPGQAGPSGPPGPPGAIGPSGPAGKDGESPGGRPGRPGERGLPGPPGIKGPAGIPGFPGMKGHRGFDGRNGEKGETGAPGLKGENGLPGENGAPGPMGPRGAPGERGRPGLPGAAGARGNDGARGSDGQPGPPGPPGTAGFPGSPGAKGEVGPAGSPGSNGAPGQRGEPGPQGHAGPPGPVGPAGKSGDRGESGPAGPAGAPGPAGSRGAPGPQGPRGDKGETGERGAAGIKGHRGFPGNPGAPGSPGPAGQQGAIGSPGPAGPRGPVGPSGPPGKDGTSGHPGPIGPPGPRGNRGERGSEGSPGHPGQPGPPGPPGAPGPCCGGVGAAAIAGIGGEKAGGFAPYYHHHHHH
[0045] Use the online design tool Jcat (http: / / www.jcat.de / ) to reverse design the gene sequence, aiming at the preferred codons required for expression in the host Escherichia coli, and remove the NdeI and XhoI restriction sites during the design process, which is beneficial to the later stage Gene manipulation, the optimized gene sequence is shown as SEQ ID NO: 3, compar...
Embodiment 2
[0055] Example 2 Construction of expression vector pACYCDuet 1-Hy726-1230
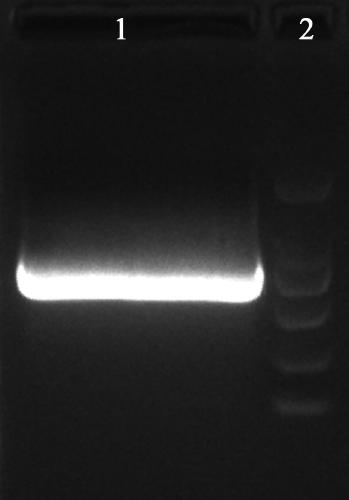
[0056] (1) Using the prokaryotic expression vector pUC-Hy726 of hydroxylase Hy726 obtained in Example 1 as a template, the Hy726 fragment was amplified and cloned by high-fidelity PCR technology, and the PCR product was purified and amplified by double digestion with Nco I and Xho I The Hy726 fragment, the gel was cut to recover Hy726 (N-X), and the results of electrophoresis separation are shown in figure 1 .
[0057] The PCR amplification system is as follows (take 50 μL as an example):
[0058] ddH2O 19.0 μL
[0059] 2 × pfu Buffer 25.0μL
[0060] Hy762 upstream primer 2.0 μL
[0061] Hy762 downstream primer 2.0 μL
[0062] Template 2.0μL (about 5ng)
[0063] Proceed as follows:
[0064] 94°C for 3 minutes
[0065] 94°C 30 sec
[0066] 55°C 30 sec
[0067] 72°C 1 min 40 sec
[0068] 72°C for 3 minutes
[0069] 35 cycles.
[0070] After the PCR product was purified, it was digested with N...
Embodiment 3
[0074] Example 3 Construction of BL21(DE3) / pACYCDuet 1-Hy726-1230
[0075] This example refers to the method of J. Sambrook et al., "Guidelines for Molecular Cloning Experiments, Third Edition", as follows: Pick a single colony of E. Add 0.5mL of overnight culture solution to a 50mL LB Erlenmeyer flask, and culture with vigorous shaking at 37°C for about 2 hours to allow the bacteria to grow to the pre-logarithmic stage; under sterile conditions, transfer the bacteria to a 50mL polypropylene tube pre-cooled with ice, Place on ice for 10min; centrifuge at 4000rpm at 4°C, pour off the supernatant, invert the tube to let the residual liquid flow out as much as possible; add 6mL of 0.1mol / L CaCl pre-cooled with ice 2 Resuspend the pellet and place it on ice for 30min; centrifuge at 3000rpm at 4°C, pour off the supernatant, invert the tube to make the residual liquid flow out as much as possible; add 1.2mL of 0.1mol / LCaCl pre-cooled with ice 2 Resuspend the pellet (if you want to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com