Preparation method of tert-butyl acrylate
A technology of tert-butyl acrylate and acrylic acid, applied in the field of preparation of tert-butyl acrylate, can solve the problems of complex polymerization inhibitor and high reaction temperature, and achieve the effects of mild reaction, simple process and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
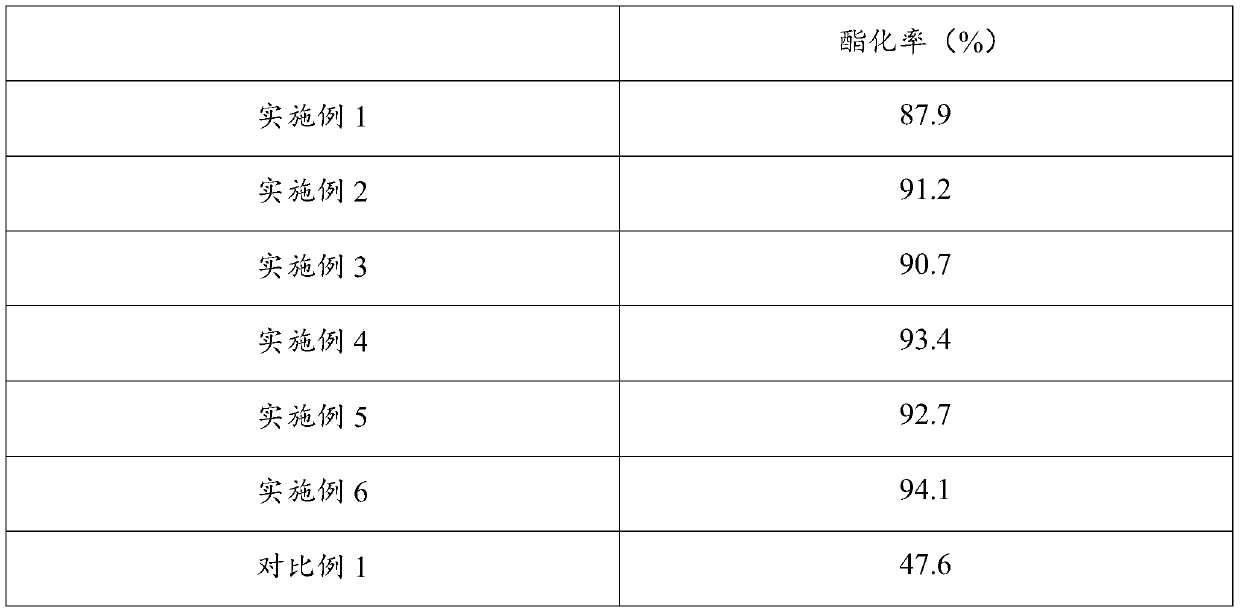
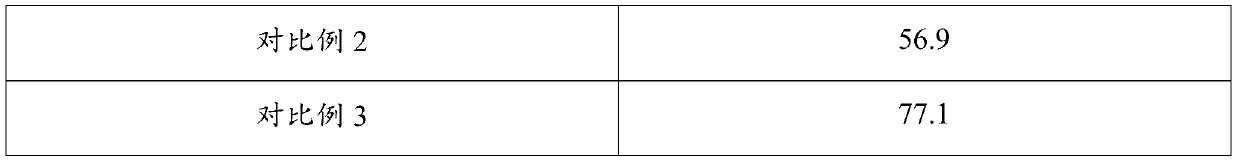
[0022] Put acrylic acid, catalyst, polymerization inhibitor and isobutylene into the reactor, under the conditions of temperature 30°C and pressure 1MPa, esterification reaction occurs at the volume space velocity of isobutylene 1.0 / h to synthesize tert-butyl acrylate;
[0023] Wherein, the molar ratio of acrylic acid, polymerization inhibitor and isobutylene is 1:0.1:0.5, the catalyst is a mixture of sulfonic acid type cation exchange resin and p-toluenesulfonic acid, the mass ratio of the two is 3:1, the total mass of the catalyst It is 10% of the mass of acrylic acid; the polymerization inhibitor is a mixture of tert-butylcatechol and tert-butanol, and the mass ratio of the two is 1:1.
Embodiment 2
[0025] Put acrylic acid, catalyst, polymerization inhibitor and isobutylene into the reactor, under the conditions of temperature 30°C and pressure 1MPa, esterification reaction occurs at the volume space velocity of isobutylene 1.0 / h to synthesize tert-butyl acrylate;
[0026] Wherein, the molar ratio of acrylic acid, polymerization inhibitor and isobutylene is 1:0.1:0.5, the catalyst is a mixture of sulfonic acid modified SBA-15 mesoporous molecular sieve and p-toluenesulfonic acid, the mass ratio of the two is 3:1, The total mass of the catalyst is 10% of the mass of acrylic acid; the polymerization inhibitor is a mixture of tert-butylcatechol and tert-butanol, and the mass ratio of the two is 1:1.
Embodiment 3
[0028] Put acrylic acid, catalyst, polymerization inhibitor and isobutylene into the reactor, under the conditions of temperature 30°C and pressure 1MPa, esterification reaction occurs at the volume space velocity of isobutylene 1.0 / h to synthesize tert-butyl acrylate;
[0029] Wherein, the molar ratio of acrylic acid, polymerization inhibitor and isobutylene is 1:0.2:0.8, the catalyst is a mixture of sulfonic acid modified SBA-15 mesoporous molecular sieve and p-toluenesulfonic acid, the mass ratio of the two is 3:1, The total mass of the catalyst is 8% of the mass of acrylic acid; the polymerization inhibitor is a mixture of tert-butylcatechol and tert-butanol, and the mass ratio of the two is 1:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com