A kind of metal porphyrin compound and its preparation method and application
A compound and reaction technology, applied in metalloporphyrin compound and its preparation method and application field, can solve problems such as lack of binding ability, and achieve the effects of simple method, good HIV reverse transcriptase inhibitory ability, and good spectral properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] This embodiment discloses the preparation of the metalloporphyrin compound of the present invention
[0050] 1. Preparation of carboxyporphyrin compound TPPCOOH1:
[0051] Weigh methyl p-formylbenzoate (9mmol, 1.49mg) and benzaldehyde (27mmol, 2.76ml) in a 250ml double-necked round bottom flask and dissolve in 120ml propionic acid. After the reaction solution was heated to 140°C, redistilled pyrrole (36mmol, 2.5ml) was added and refluxed for two hours. After the reaction solution was cooled to room temperature, 20ml of ethanol was added. Place in the refrigerator to cool overnight, a purple solid was formed, and after suction filtration and drying, column chromatography was performed with a 2:1 mixed solvent of dichloromethane and petroleum ether to obtain 1.26 g of methyl tetraphenylporphyrin carboxylate. Dissolve the product in 80ml of tetrahydrofuran, then add 160ml of 1mol / L potassium hydroxide solution, and place it under reflux at 66°C for 72h. After the reaction...
Embodiment 2
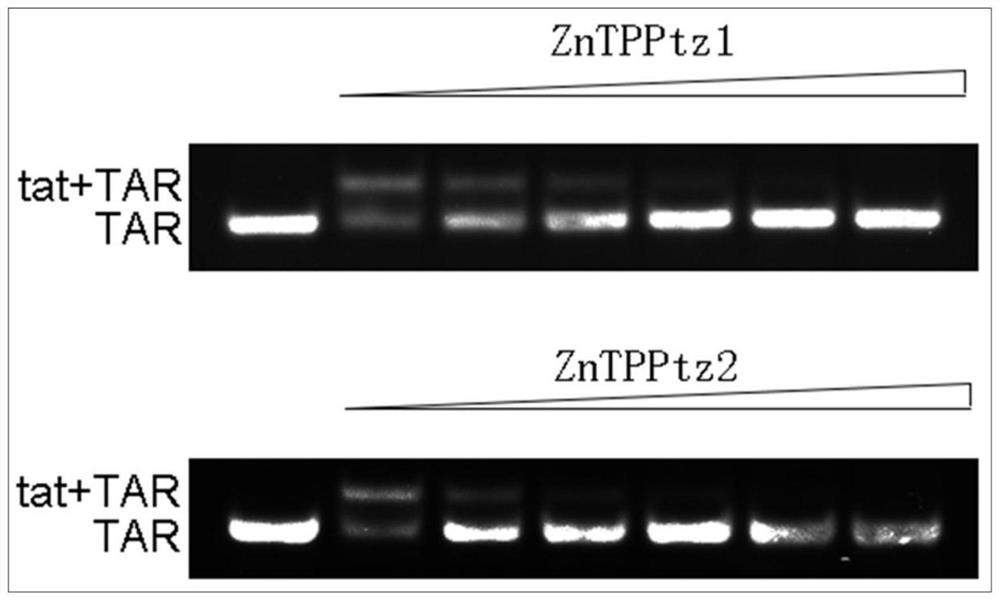
[0064] This example discloses an electrophoresis experiment in which the metalloporphyrin compound of the present invention recognizes HIV TAR RNA.
[0065] HIV TAR RNA (Shanghai Sangon Biotech) and TAR-binding protein tat (Shanghai Qiangyao Biology) were prepared in a 0.1mM solution (50mM Tris buffer, pH 7.3). Add 4 μl TARRNA and 4 μl TAT to the reaction tubes, and incubate at 37°C for 1 hour. Add 12 μl of water or different concentrations of metalloporphyrin compounds to prepare a reaction solution with a total volume of 20 μl, and incubate at 37° C. for 1 hour. Take 8 μl of the above reaction solution, add 2 μl bromophenol blue loading buffer, and conduct polyacrylamide gel electrophoresis experiment. 4S Gel Red staining, gel imaging on FluorChemFC3. The result is as figure 1 As shown, the concentration of metalloporphyrin compound is 0, 5, 10, 20, 40, 80μM from left to right. As the concentration of metalloporphyrin compound increases, the TAR RNA bound to tat protein d...
Embodiment 3
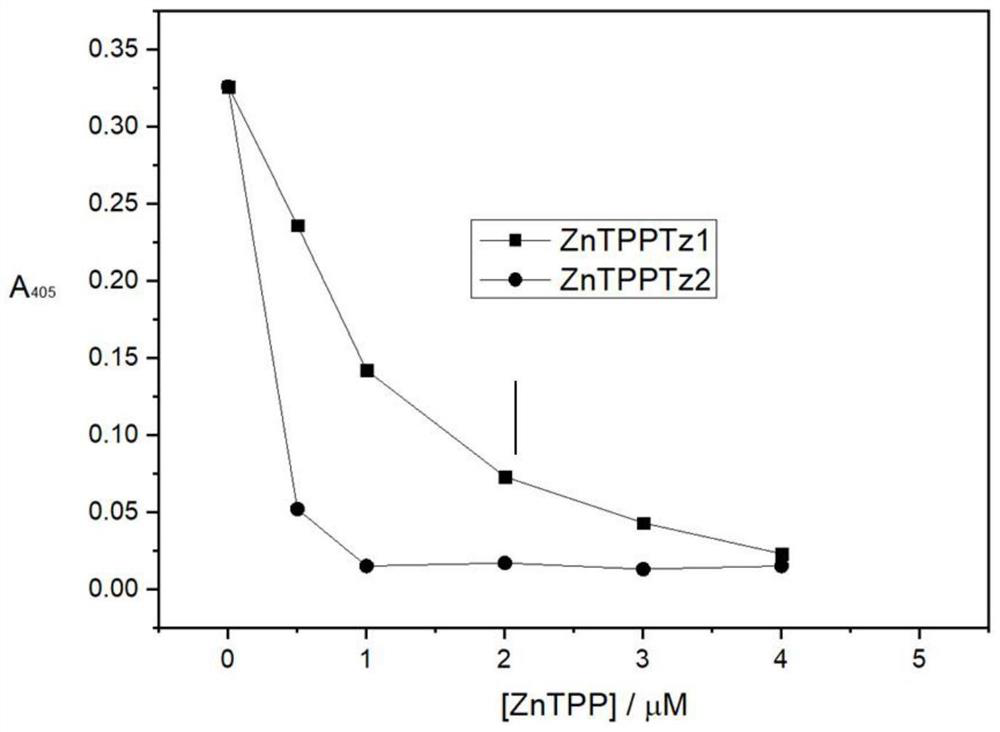
[0067] This example discloses the enzyme-linked immunosorbent assay of the metalloporphyrin compound of the present invention inhibiting HIV reverse transcriptase.
[0068]Using the Roche kit (Reverse Transcriptase Assay, colorimetric), the inhibitory activity of the metalloporphyrin compound prepared in the present invention on the recombinant HIV-1 HIV reverse transcriptase was tested based on an enzyme-linked immunosorbent assay. In a separate reaction tube, 5 ng of recombinant HIV-1 HIV reverse transcriptase was added and diluted in lysis buffer (20 μl / well). Lysis buffer without added recombinant HIV-1 HIV reverse transcriptase should be used as a negative control. Add 20 μl of metalloporphyrin diluted in lysis buffer and 20 μl of reaction mixture (46 mM Tris, 266 mM KCl, 27.5 mM MgCl, 9.2 mM dithiothreitol, 10 μM dUTP / dTTP, 0.75 A 260 / ml template poly(A) / primer(dT) 15 ), and incubated at 37°C for 1 hour. The above samples (60 μl) were transferred to enzyme-labeled st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com