Ni/la for hydrogen production by autothermal reforming of acetic acid 2 x 2 o 7 catalyst
An autothermal reforming and catalyst technology, applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, heterogeneous catalyst chemical elements, etc. Oxidation, poor stability and other problems, to achieve the effect of increasing fluidity, improving oxygen transfer capacity, and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
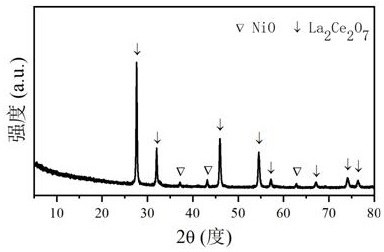
[0029] Take 5.170g of La(NO 3 ) 3 ·6H 2 O, 5.184g of Ce(NO 3 ) 3 ·6H 2 O, 3.011g of citric acid, add 50mL of deionized water, then stir until a clear solution is formed; slowly add NH 3 ·H 2 O solution was adjusted to its pH value to 2, and then stirred continuously in a water bath at 80 °C until a viscous gel was formed; the gel was aged at 130 °C for 12 h, and then heated in a resistance furnace at a rate of 10 °C / min to 800°C and roasted at this temperature for 4h, the catalyst precursor was obtained; 2.531g of Ni(NO 3 ) 2 ·6H 2 O, add 50mL of deionized water to make a solution, impregnate the catalyst precursor, and then dry at 80°C for 10h; in a resistance furnace at a heating rate of 10°C / min to 800°C and bake at this temperature for 4h , to obtain CDUT-LX-1 catalyst, its crystal structure is attached figure 1 As shown, the La 2 Ce 2 o 7 Loaded dispersed NiO phase, after reduction to form Ni / La 2 Ce 2 o 7 active center structure. The molar composition of...
Embodiment 2
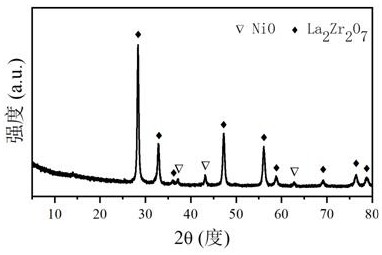
[0033] Take 6.054g of La(NO 3 ) 3 ·6H 2 O, 3.736g of ZrO(NO 3 ) 2 2H 2 O, 3.525g of citric acid, add 50mL of deionized water, then stir until a clear solution is formed; slowly add NH 3 ·H 2 O solution was adjusted to its pH value to 2, and then stirred continuously in a water bath at 80 °C until a viscous gel was formed; the gel was aged at 130 °C for 12 h, and then heated in a resistance furnace at a rate of 10 °C / min to 800°C and roasted at this temperature for 4h, the catalyst precursor was obtained; 2.747g of Ni(NO 3 ) 2 ·6H 2 O, add 50mL deionized water to prepare a solution, impregnate the catalyst precursor, and then dry at 80°C for 10h; in a resistance furnace at a heating rate of 10°C / min to 800°C and bake at this temperature for 4h , to obtain CDUT-LX-2 catalyst, its crystal structure is attached figure 2 As shown, the cubic pyrochlore crystal phase La is formed 2 Zr 2 o 7 Loaded dispersed NiO phase, after reduction to form Ni / La 2 Zr 2 o 7 active c...
Embodiment 3
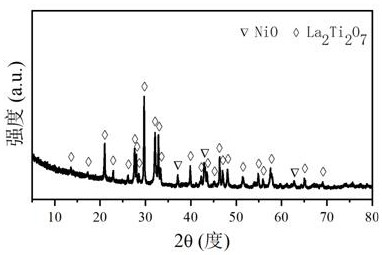
[0036] Take 7.137g of La(NO 3 ) 3 ·6H 2 The citric acid of O, 4.156g is added the deionized water of 50mL, is mixed with solution #1; Get 5.609g of Ti(OC 4 h 9 ) 4 Add it into an appropriate amount of nitric acid solution to prepare solution #2; mix solution #1 and solution #2 to obtain solution #3, keep stirring until clear; slowly add NH 3 ·H 2 O solution was adjusted to its pH value to 2, and then stirred continuously in a water bath at 80 °C until a viscous gel was formed; the gel was aged at 130 °C for 12 h, and then heated in a resistance furnace at a rate of 10 °C / min to 800°C and roasted at this temperature for 4h, the catalyst precursor was obtained; 2.748g of Ni(NO 3 ) 2 ·6H 2 O was added to 50 mL of deionized aqueous solution, impregnated onto the catalyst precursor, and then dried at 80 °C for 10 h; after calcination at 800 °C at a heating rate of 10 °C / min in a resistance furnace for 4 h, the CDUT- LX-3 catalyst, its crystal structure is attached image ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com