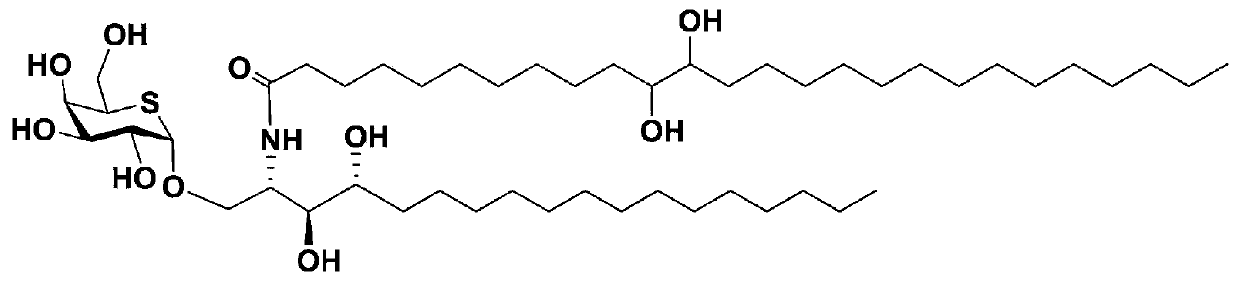
Glycoside analogue, and preparation method and application thereof
A technology of compounds and glycosides, which is applied in the fields of chemistry and medicine, can solve the problems of poor activity of compounds, and achieve the effects of simple conventional treatment, high yield, and easy control of the reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The present invention also relates to the preparation method of the above-mentioned glycoside compound, which is completed through the following two synthesis steps:
[0036] (1) Preparation of o-dihydroxy compound 6:
[0037] The following synthetic scheme describes the synthetic method for the preparation of the ortho-dihydroxy compound 6:
[0038]
[0039] Wherein, reagent and reaction condition in the above-mentioned synthetic method: (a) (i) Dess-Martin reagent, dichloromethane; (ii) C 14 h 27 Ph 3 P + Br - (tetradecyl quaternary phosphonium salt), LHMDS (lithium hexamethyldisilazide), tetrahydrofuran; (b) AD-Mix-β, p-methanesulfonamide; (c) 2,2-dimethoxytoluene , tetrahydrofuran; (d) lithium hydroxide, tetrahydrofuran - methanol - water system.
[0040] (2) Preparation of glycoside compound 1:
[0041] The following synthetic scheme describes the synthetic method for the preparation of glycoside compound 1:
[0042]
[0043] Among them, the reagents ...
Embodiment 1
[0049] Preparation of Glycoside Compound 1
[0050]
[0051] 1. Preparation of o-dihydroxy compound 6:
[0052]
[0053] Starting from the known compound 2, we oxidized the primary hydroxyl group to form an aldehyde through Dess-Martin, and then extended the carbon chain to the designed carbon chain length containing an unsaturated double bond through the Wittig reaction. This double bond is exactly the position of the designed double hydroxyl group. , the double bond was subjected to Sharpless dihydroxylation, followed by condensation reaction with benzaldehyde to complete the protected carboxylic acid compound 6. The specific operation process is as follows:
[0054] Compound 3: Add Dess-Martin reagent (746mg, 1.76mmol) to compound 2 (311mg, 1.35mmol) dissolved in dichloromethane (10mL) at zero temperature, and react for 6 hours. -78°C 14 h 29 Ph 3 P + Br - (800mg, 1.48mmol) tetrahydrofuran solution, and then slowly added LHMDS (2.5M in THF, 0.59ml, 1.48mmol) to...
Embodiment 2
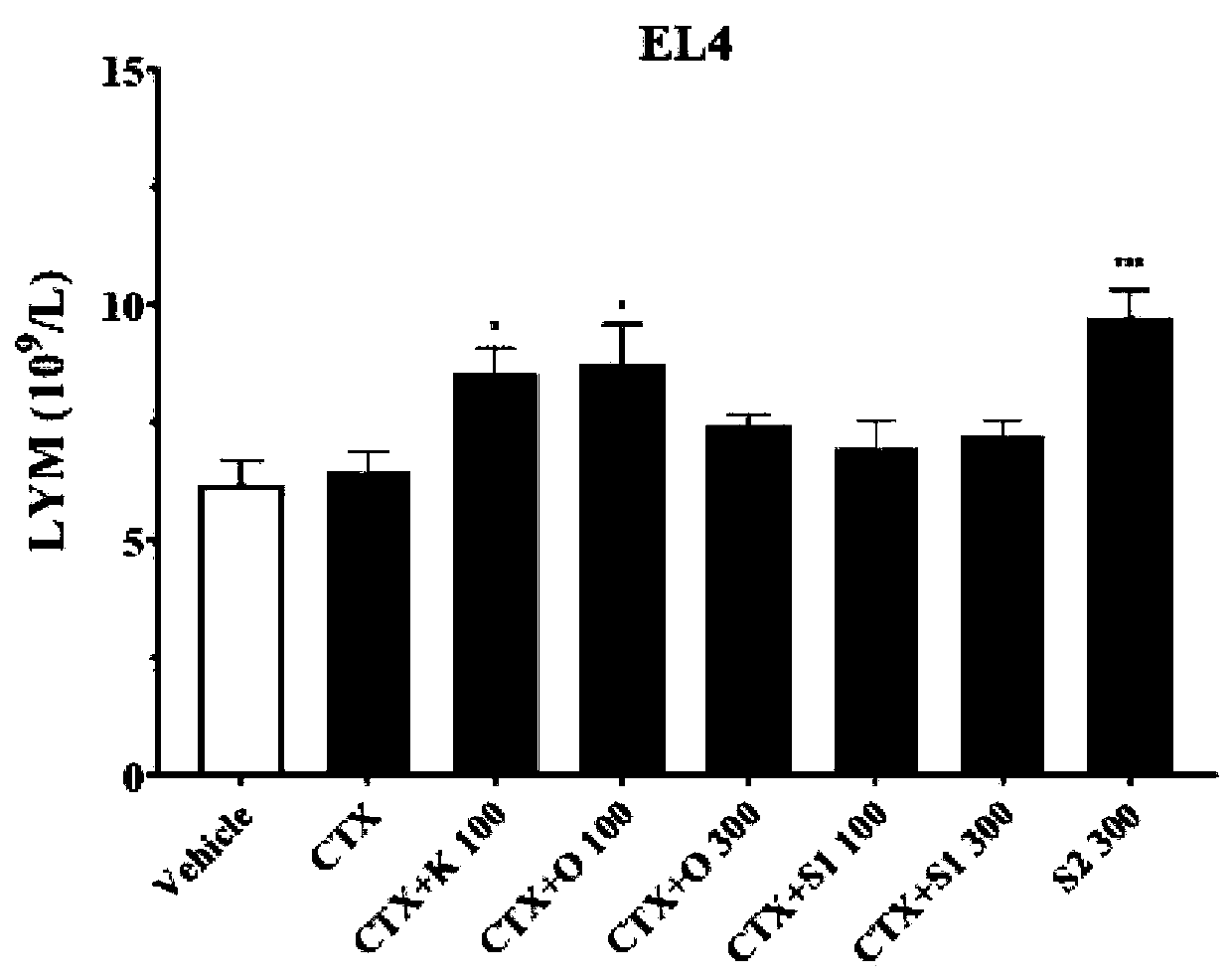
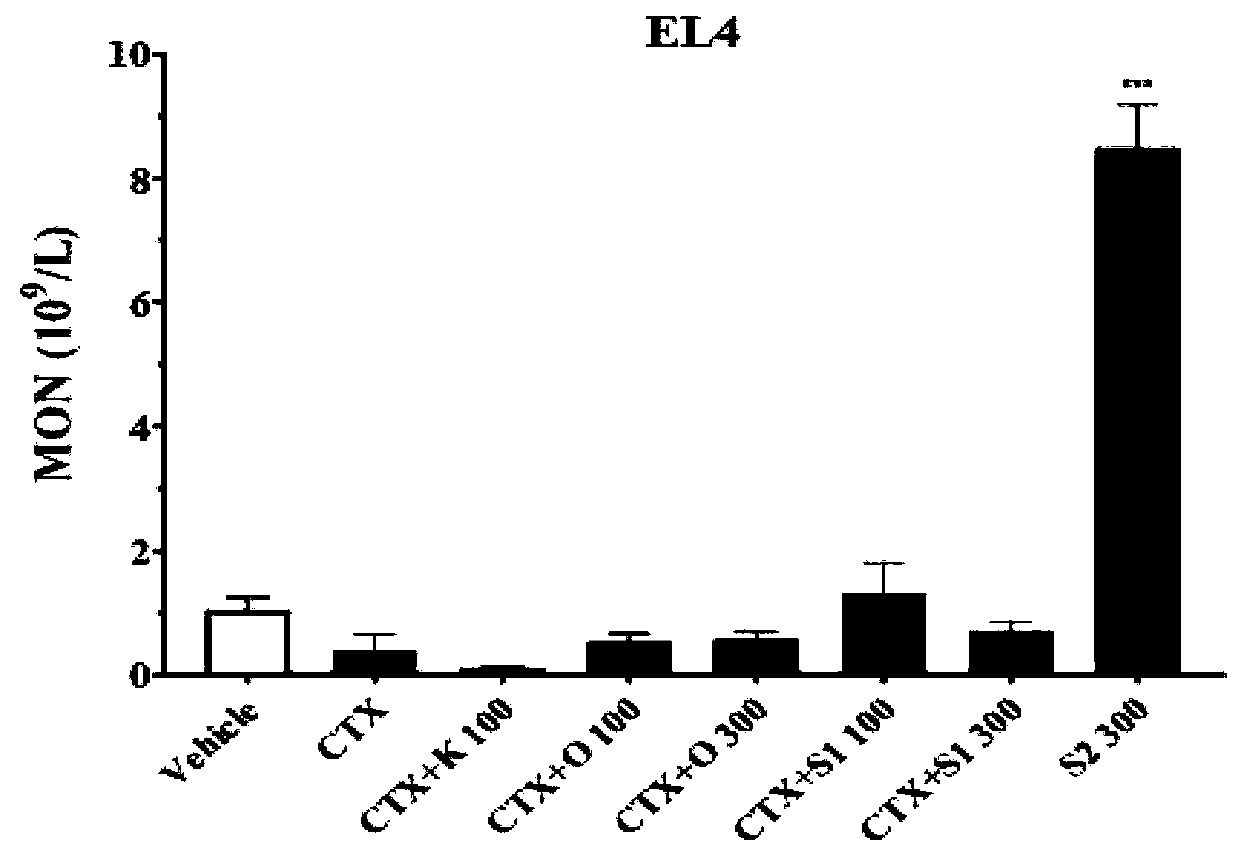
[0072] Evaluation of Inhibition Rate of Glycoside Compound 1 on Lymphoma
[0073] Biological experiments
[0074] Aseptically place 1 x 10 7 Mouse T-cell lymphoma EL4 cells were inoculated into the axilla of C57BL / 6 mice. After 10 days, the tumor tissue was obtained under sterile conditions, and the homogenate was diluted with normal saline and counted to make a concentration of 1×10 7 cells / mL tumor cell suspension, inoculate 0.2 mL / mouse in the right armpit of mice. Animals were randomly divided into groups before inoculation, with 8 animals in each group. Glycoside compound 1 (denoted as Day-1) was administered one day before modeling, administered once on the same day as cyclophosphamide (CTX) on the first day after modeling, and then three days later. Administer once. After 2 weeks, the body weight was measured, the animals were sacrificed, the tumor tissues were stripped, weighed and photographed. Finally, the tumor inhibition rate was calculated, and the effect str...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com