Gardenia polysaccharide and preparation method and application thereof
A kind of gardenia polysaccharide, gardenia technology, applied in pharmaceutical formulations, medical preparations containing active ingredients, digestive system and other directions, can solve the problem of limited research and development, no liver protection ingredients, can not fully represent the gardenia jaundice and liver protection, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation method of gardenia polysaccharide, the step comprises:
[0037] Gardenia medicinal materials were sun-dried, crushed and passed through an 80-mesh sieve to obtain Gardenia powder; the Gardenia powder was soaked in ethanol for 24 hours to degrease, filtered, and the dregs were dried in the air, wherein the solid-to-liquid ratio of Gardenia powder and ethanol was 1g:8mL; Extract the dregs with 100°C hot water 20 times the mass of the dregs for 3 times, 3 hours each time, filter, combine the filtrate, concentrate the filtrate to 1 / 4 of the original filtrate, and obtain the concentrated solution A, which is 3500×g Centrifuge for 10 minutes, take the supernatant to obtain the water extract; add ethanol to the water extract to make the alcohol concentration reach 70%, let it stand overnight at room temperature, filter and collect the precipitate, further soak the precipitate twice with 95% absolute ethanol, and then place it at 40°C Oven for 20 minutes, then a...
Embodiment 2
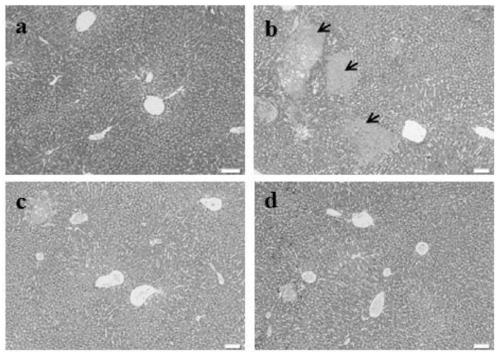
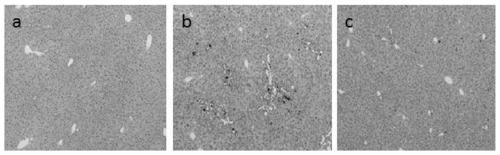
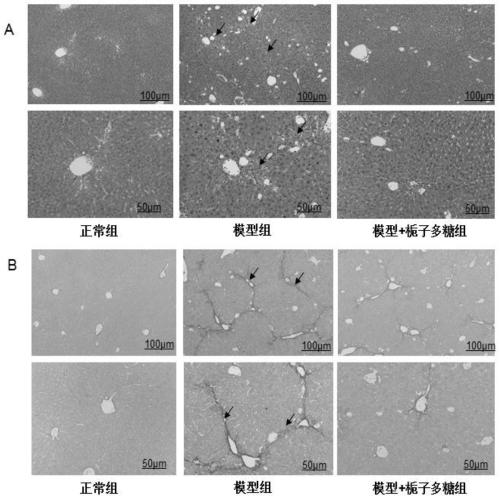
[0040]Healthy male C57BL / 6J mice, weighing 20±2g, were provided by Shanghai Lingchang Animal Co., Ltd. After one week of adaptive feeding, the mice were randomly divided into normal control group, model control group, ursodeoxycholic acid positive control group, and gardenia polysaccharide group, with 10 mice in each group. The positive control group was given 115 mg / kg of ursodeoxycholic acid by intragastric administration, the normal control group and the model control group were given intragastric administration of equal volume of normal saline, and the gardenia polysaccharide group was given 400 mg / kg of gardenia polysaccharide (dissolved in normal saline) by intragastric administration. Once a day, continuous administration for 14 days. After 4 hours of administration on the 12th day of the test, except the normal control group, the other groups were intragastrically administered α-naphthalene isothiocyanate (ANIT) 50 mg / kg to replicate the acute cholestatic liver injury ...
Embodiment 3
[0052] Healthy male C57BL / 6J mice, weighing 22±2g, were provided by Beijing Weitong Lihua Experimental Animal Co., Ltd. After one week of adaptive feeding, the mice were randomly divided into normal control group, model control group and gardenia polysaccharide group. Except for the normal control group given normal feed, other groups were given 0.025% 3,5-diethoxycarbonyl-1,4-dihydro-2,4,6-collidine (3,5-diethoxycarbonyl- 1,4-dihydroxychollidine, DDC) toxic feed, to reproduce the chronic cholestasis liver injury model, 1 week after the model induction, the gardenia polysaccharide group was given 800mg / kg gardenia polysaccharide (dissolved in normal saline), and the normal group and the model The rats in the group were given the same amount of normal saline by intragastric administration, once a day. During the administration period, the general symptoms of the animals during the experiment were closely observed. After 4 weeks of drug intervention (5 weeks after DDC modeling)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com