Zinc-based catalyst used for catalyzing acetylene hydration reaction and preparation method thereof
A technology of acetylene hydration and catalyst, which is applied in the direction of catalyst activation/preparation, carbon-carbon triple bond hydration preparation, physical/chemical process catalysts, etc. It can solve the problems of poor stability, high energy consumption, and low selectivity, and achieve improved product quality. The effect of high performance, small particles, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The specific operation steps are as follows:
[0036] (1) Preparation of precursor solution: 2 g of zinc chloride was completely dissolved in 18.0 mL of deionized water to obtain a precursor solution;
[0037] (2) Prepare the catalyst: add the precursor solution dropwise to the molecular sieve carrier, stir for 12 hours, let stand for 12 hours, and dry at 100° C. for 18 hours to obtain the catalyst, namely Zn / MCM-41 catalyst.
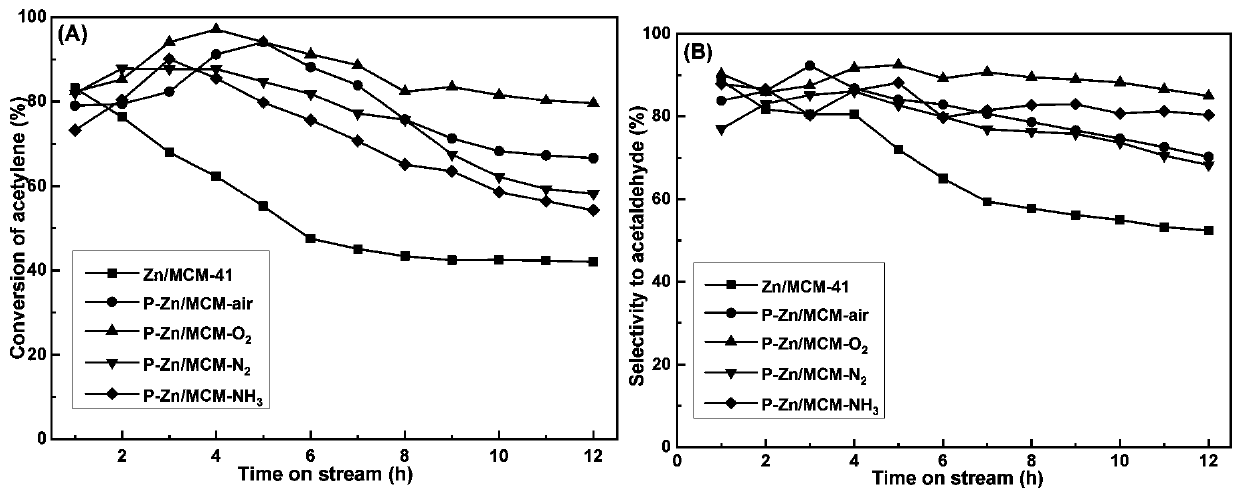
[0038](3) Plasma treatment: the prepared catalyst is processed under nitrogen, ammonia, oxygen and air plasma atmosphere respectively, the treatment condition is that the time is 30min, the power is 100w, and the gas flow is 10mL / min, to obtain the described Zinc-based catalysts for catalyzing the hydration of acetylene, namely P-Zn / MCM-N 2 , P-Zn / MCM-NH 3 , P-Zn / MCM-O 2 , P-Zn / MCM-air.
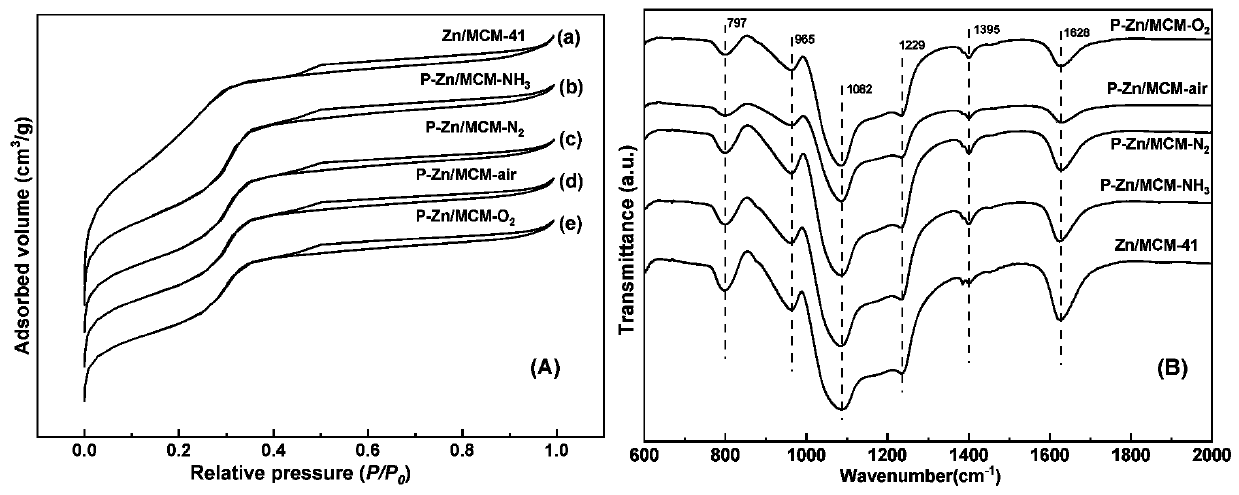
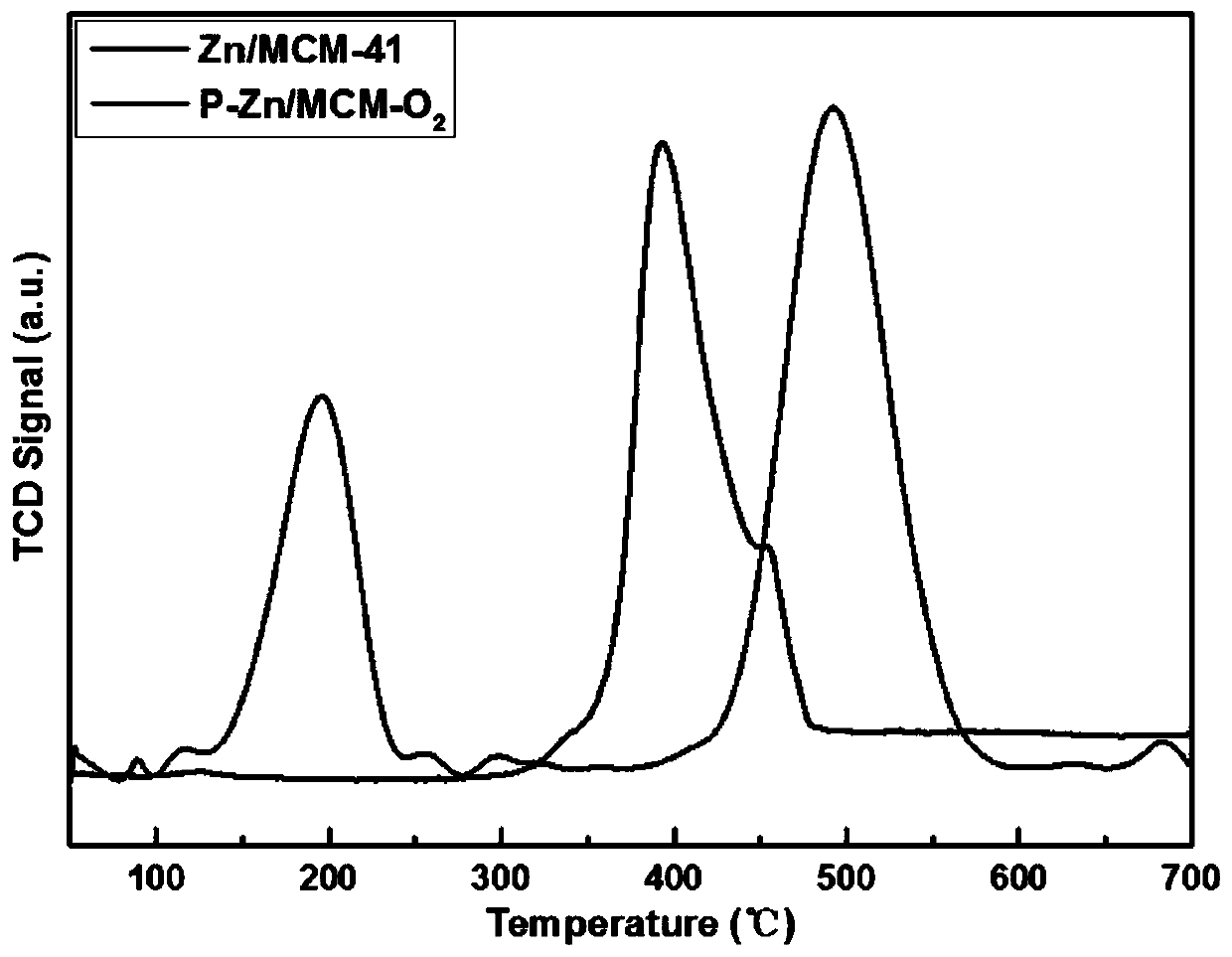
[0039] In order to more vividly describe the structure of the plasma-treated catalyst prepared under different atmospheres, we carried out N 2 Adsorption a...
Embodiment 2
[0045] The specific operation steps are as follows:
[0046] (1) Preparation of precursor solution: 2 g of zinc chloride was completely dissolved in 18.0 mL of deionized water to obtain a precursor solution;
[0047] (2) Prepare the catalyst: add the precursor solution dropwise to the molecular sieve carrier, stir for 12 hours, let stand for 12 hours, and dry at 100° C. for 18 hours to obtain the catalyst, namely Zn / MCM-41 catalyst.
[0048] (3) Plasma treatment: the prepared zinc-based catalyst is treated under an oxygen plasma atmosphere, the treatment conditions are that the power is 100w, the gas flow rate is 10mL / min, and the time is 30min, 60min, 90min, 120min respectively, depending on Zinc-based catalysts for the hydration of acetylene by temporal oxygen plasma treatment.
[0049] During the activity test, when the reaction temperature is 100-400°C, C 2 h 2 The flow rate is 3.4mL / min, H 2 The flow rate of O is 0.01g / min, and the space velocity is adjusted to 90h -...
Embodiment 3
[0051] The specific operation steps are as follows:
[0052] (1) Preparation of precursor solution: 2 g of zinc chloride was completely dissolved in 18.0 mL of deionized water to obtain a precursor solution;
[0053] (2) Prepare the catalyst: add the precursor solution dropwise to the molecular sieve carrier, stir for 12 hours, let stand for 12 hours, and dry at 100° C. for 18 hours to obtain the catalyst, namely Zn / MCM-41 catalyst.
[0054] (3) Plasma treatment: The prepared zinc-based catalyst was treated in an oxygen plasma atmosphere. The treatment conditions were 30 minutes, a gas flow rate of 10 mL / min, and a power of 50w, 75w, and 100w to obtain different oxygen plasmas. Zinc-based catalysts for the hydration of acetylene for bulk discharge power processing.
[0055] During the activity test, when the reaction temperature is 100-400°C, C 2 h 2 The flow rate is 3.4mL / min, H 2 The flow rate of O is 0.01g / min, and the space velocity is adjusted to 90h -1 Under the con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com