Mn<4+> doped red fluorescent material and preparation method thereof
A technology for red fluorescence and raw materials, applied in the field of Mn4+-doped red fluorescent materials and their preparation, can solve the problems of low light emission color rendering index of white LEDs, harm to human health and environment, poor chemical stability, etc., and achieve enhanced photoluminescence. , low cost, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
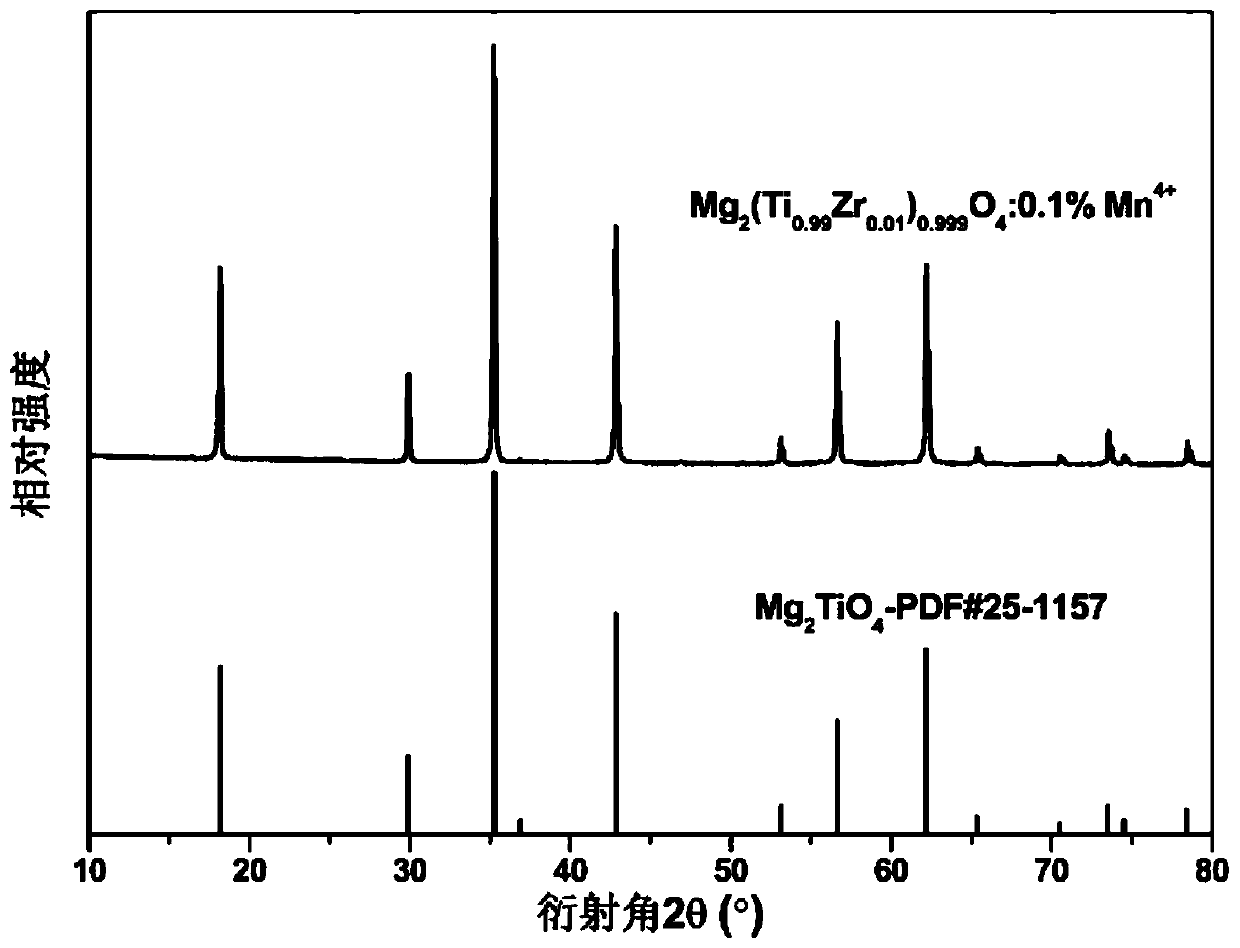
[0032] Embodiment 1: adopt high-temperature solid phase method to prepare (Mg 1-y A y ) 2 (Ti 1-z B z ) 1-x o 4 :xMn 4+ (B=Zr; x=0.001, y=0, z=0.01), the raw materials used are analytically pure MgO, TiO 2 , ZrO 2 and MnO 2 ; Then accurately weigh the raw materials and add 5wt% NH according to the molar ratio in the chemical reaction equation 4 F flux, grind and mix evenly with an agate mortar to obtain a precursor; put the precursor into a 25×25mm alumina crucible, then place it in a muffle furnace, and heat it up to 800°C in an air atmosphere for pre-sintering. Insulate for 6 hours, take it out and re-grind it to make it evenly mixed, and heat it for 10 hours under the condition of air atmosphere and 1400°C for the second sintering, put the obtained sample in an agate mortar and grind it, and finally get Mg 2 (Ti 0.99 Zr 0.01 ) 0.999 o 4 :0.1%Mn 4+ Phosphor. X-ray powder diffraction (XRD) analysis result shows, the XRD pattern of sample and M g2 TiO 4 Stand...
Embodiment 2
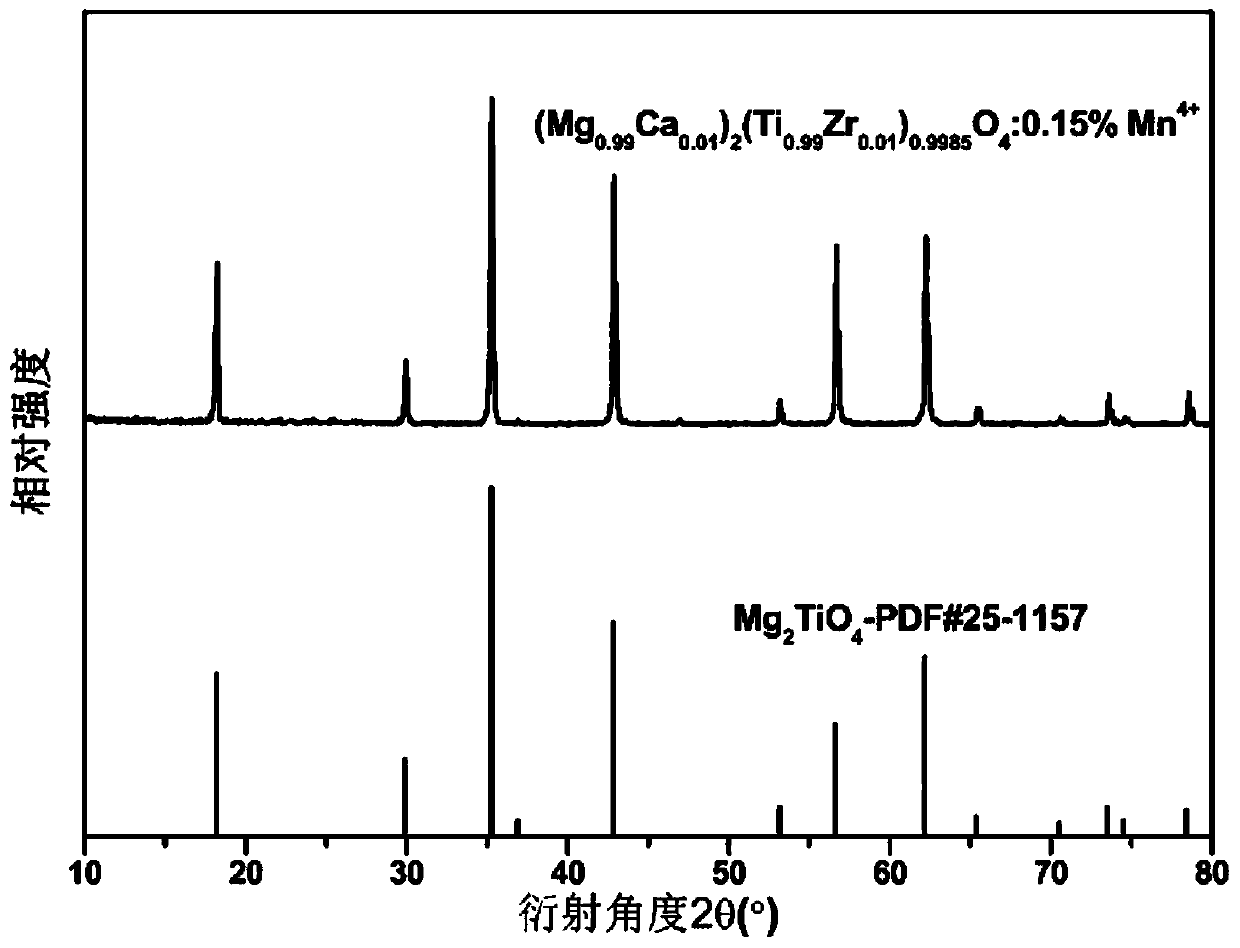
[0033] Embodiment 2: adopt high temperature solid state method to prepare (Mg 1-y A y ) 2 (Ti 1-z B z ) 1-x o 4 :xMn 4+ (A=Ca, B=Zr; x=0.0015, y=0.01, z=0.01), the raw materials used are analytically pure MgO, CaCO 3 、TiO 2 , ZrO 2 and MnO 2 Then, according to the proportion of the chemical reaction equation, accurately weigh the raw materials and add 3wt% LiF flux, grind and mix them evenly with an agate mortar to obtain a precursor, put the precursor into a 25×25mm alumina crucible, and then place it in In the muffle furnace, the temperature is raised to 600°C in the air atmosphere for pre-sintering, and the temperature is kept for 8 hours. After taking it out, it is re-grinded to make it evenly mixed. The samples were ground in an agate mortar to obtain (Mg 0.99 Ca 0.01 ) 2 (Ti 0.99 Zr 0.01 ) 0.9985 o 4 :0.15%Mn 4+ Phosphor. The X-ray powder diffraction analysis results show that the XRD pattern of the sample is consistent with M g2 TiO 4 The standard P...
Embodiment 3
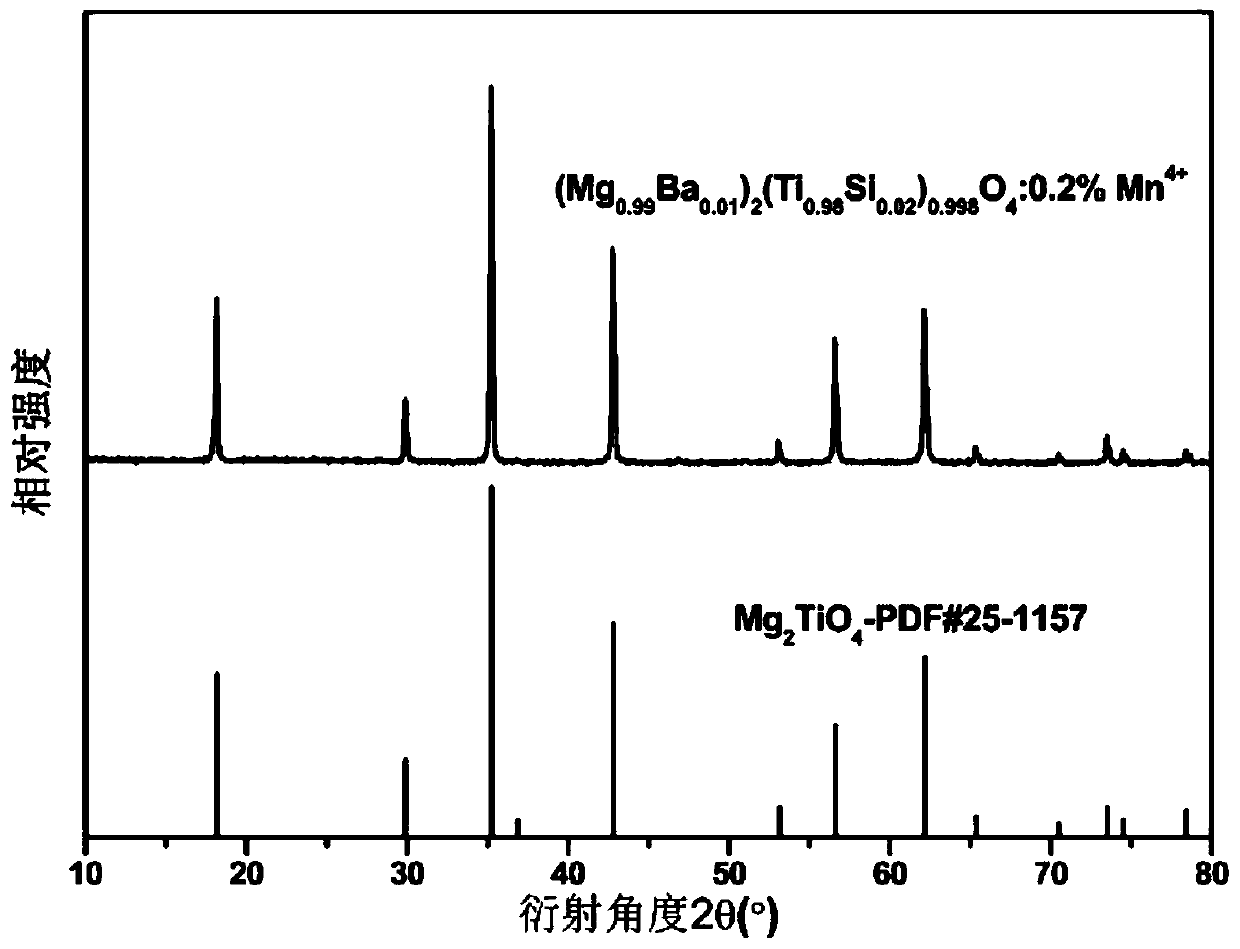
[0034] Embodiment 3: adopt high-temperature solid phase method to prepare (Mg 1-y A y ) 2 (Ti 1-z B z ) 1-x o 4 :xMn 4+ (A=Ca, B=Si; x=0.001, y=0.01, z=0.005), the raw materials used are analytically pure MgO, CaCO 3 、TiO 2 , SiO 2 and MnO2 Then, according to the proportion of the chemical reaction equation, accurately weigh the raw materials and add 5wt% LiF flux, grind and mix them evenly with an agate mortar to obtain a precursor, put the precursor into a 25×25mm alumina crucible, and then place it in In the muffle furnace, the temperature is raised to 600°C in the air atmosphere for pre-sintering, and the temperature is kept for 8 hours. After taking it out, it is re-ground to make it evenly mixed. The second sintering is held in the air atmosphere and 1150°C for 8 hours. The sample was placed in an agate mortar and ground to obtain (Mg 0.99 Ca 0.01 ) 2 (Ti 0.995 Si 0.005 ) 0.999 o 4 :0.1%Mn 4+ Phosphor. X-ray powder diffraction analysis results show that...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com