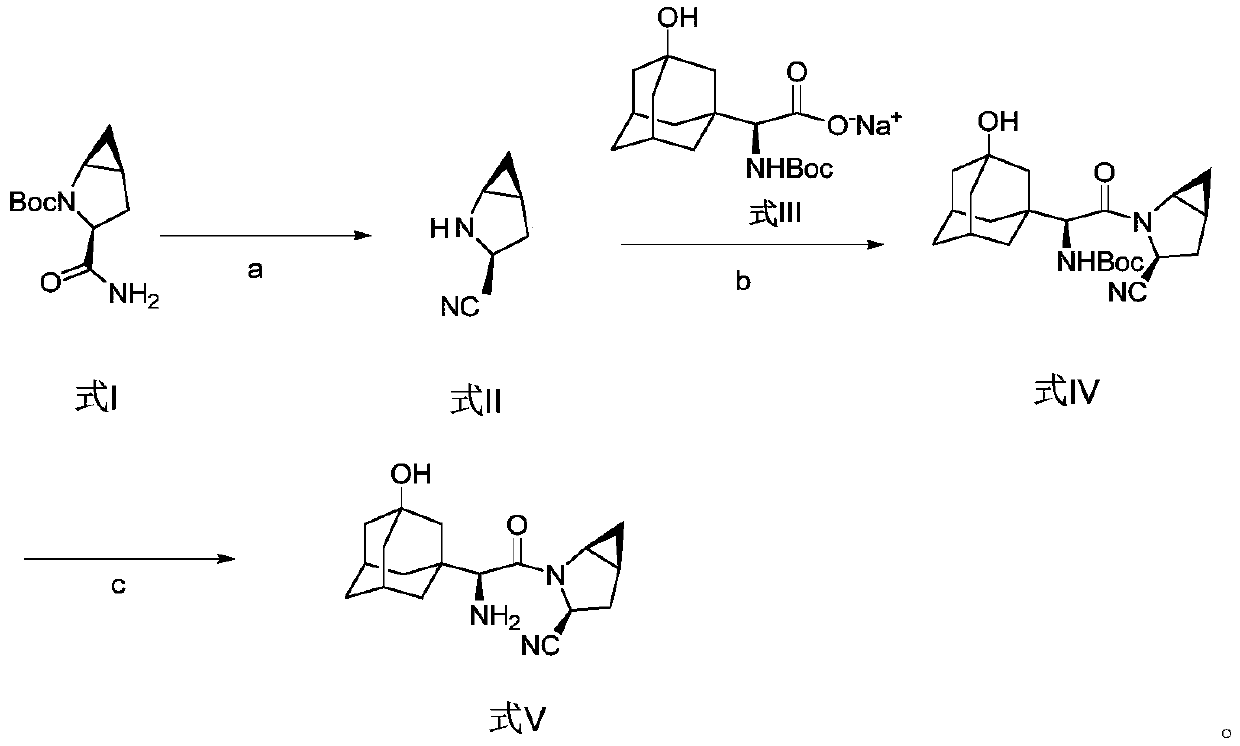
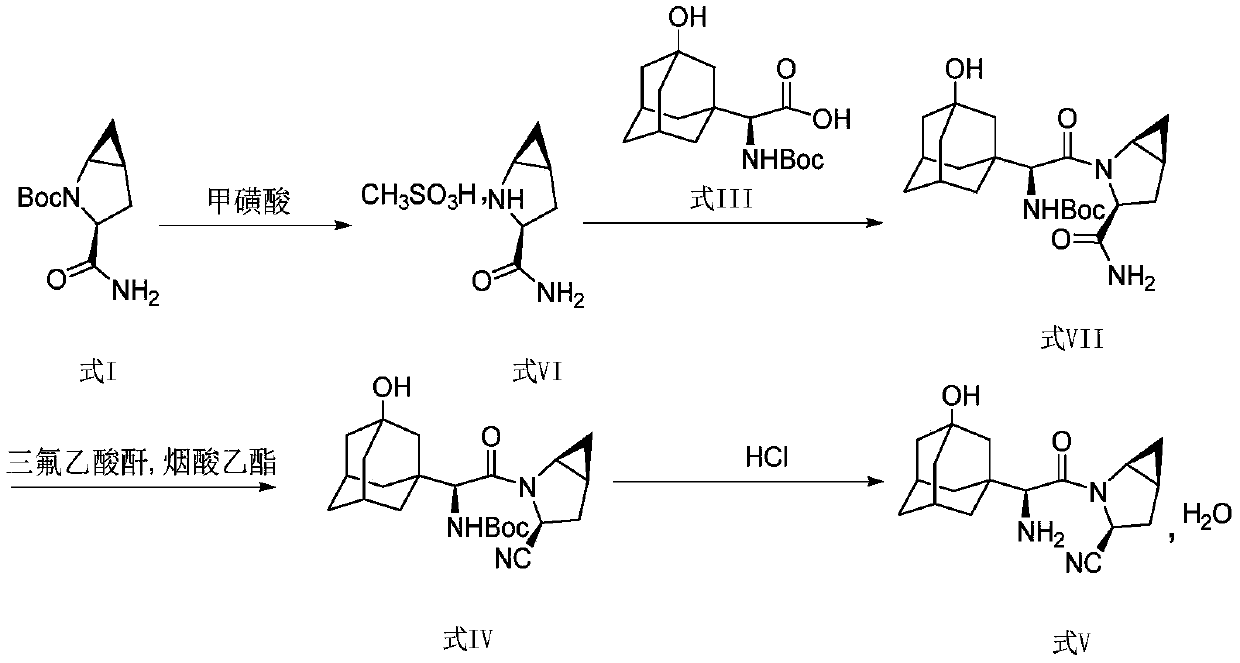
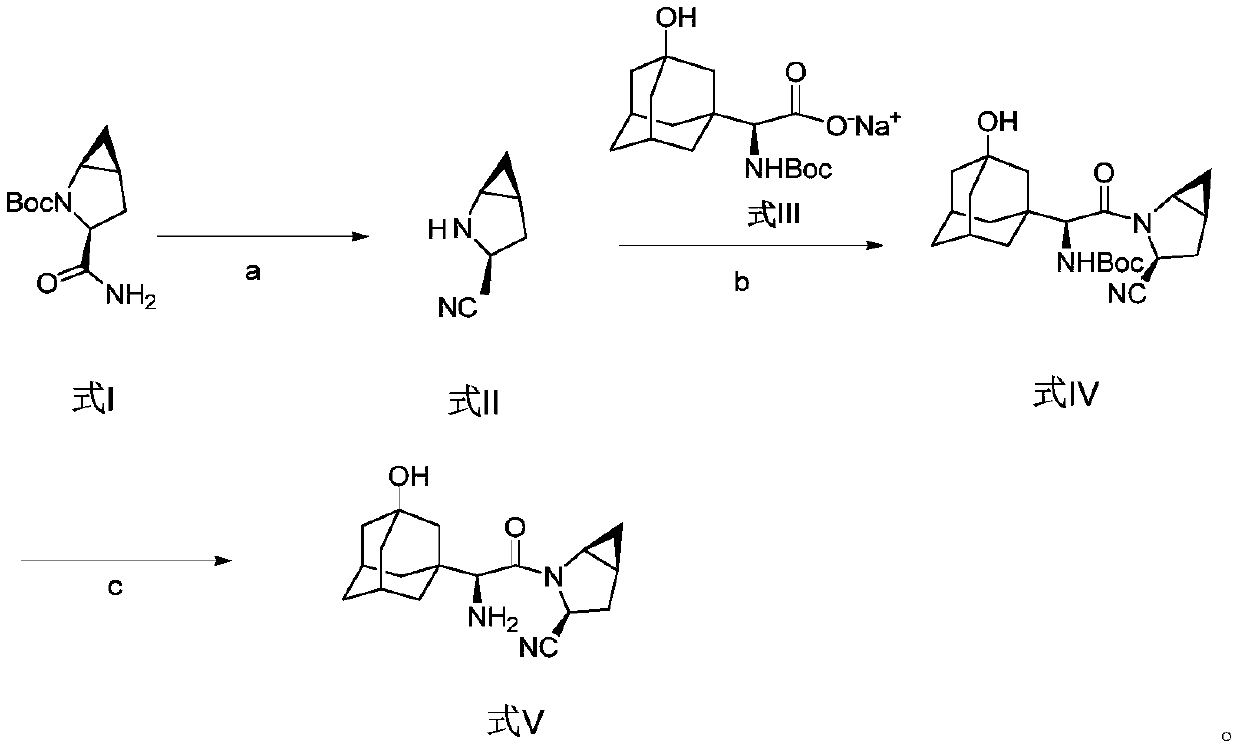
Method for preparing saxagliptin
A technology of compound and condensing agent, which is applied in the field of preparation of saxagliptin, can solve the problems that it is not suitable for industrial operation, difficult to remove ethyl nicotinate, etc., achieve good economic benefits, reduce the requirements of reaction conditions, and simplify the process steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] At room temperature, the compound of formula I (22.63g, 0.10mol) was dissolved in 75ml of ethyl acetate, pyridine (18.98g, 0.24mol) and benzenesulfonyl chloride (17.66g, 0.10mol) were added, and the reaction was stirred for 3 hours, Generate the compound of formula II, add hydrochloric acid solution (50ml, 3mol / L), stir and separate layers, extract the aqueous layer with dichloromethane (60ml×3), combine the organic phases, concentrate under reduced pressure, then place the organic phase concentrate in hydrochloric acid (50ml, 3mol / L) and diethyl ether (300ml) mixed solution, stirred for 8 hours, and the solvent was removed by distillation under reduced pressure, and the solid residue was redissolved in anhydrous ether, filtered and washed with anhydrous ether, and vacuum-dried. 12.11 g of the compound of formula II was obtained with a molar yield of 98.3% and a purity of 99.50%.
Embodiment 2
[0047]At room temperature, the compound of formula I (18.10 g, 0.08 mol) was dissolved in 75 ml of ethyl acetate, pyridine (15.82 g, 0.20 mol) and benzenesulfonyl chloride (14.13 g, 0.08 mol) were added, and the reaction was stirred for 3 hours, Generate the compound of formula II, add hydrochloric acid solution (50ml, 3mol / L), stir and separate layers, extract the aqueous layer with dichloromethane (60ml×3), combine the organic phases, concentrate under reduced pressure, then place the organic phase concentrate in hydrochloric acid (50ml, 3mol / L) and diethyl ether (300ml) mixed solution, stir 8 hours, adopt decompression distillation to remove solvent, solid residue is dissolved in ethyl acetate, obtain 30ml formula II compound ethyl acetate solution, set aside;
[0048] Dissolve the compound of formula III (34.74g, 0.10mol) in 75ml of acetonitrile, dissolve 20.7g of DIEA in 50ml of ethyl acetate, add the mixed solution of DIEA / ethyl acetate to the acetonitrile solution of the...
Embodiment 3
[0050] Take by weighing 20g of the compound of formula IV prepared in Example 2, then dissolve it in the mixed solution formed by water and isopropanol, add hydrochloric acid (20ml, 3mol / L) dropwise at 55°C, finish the dropwise addition in 5min, and keep the temperature Stir the reaction at 60°C for 2 hours, then add water, adjust the pH value of the mixture to 9-10 through sodium hydroxide and potassium carbonate solution, extract with dichloromethane (100ml×3), collect the organic phase, and concentrate under reduced pressure , to obtain 20.18 g of the compound of formula V, namely saxagliptin, with a molar yield of 96.7% and an HPLC purity of 99.85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com