A kind of amphiphilic block copolymer, absorbable bone wax and preparation method
A block copolymer and amphiphilic technology, applied in drug delivery, surgery, surgical adhesives, etc., can solve problems such as unsatisfactory hemostatic effect, poor natural material source and process stability, immunogenic allergies, etc., to improve safety Sexuality and handling properties, rapid disintegration and degradation, and the effect of reducing cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
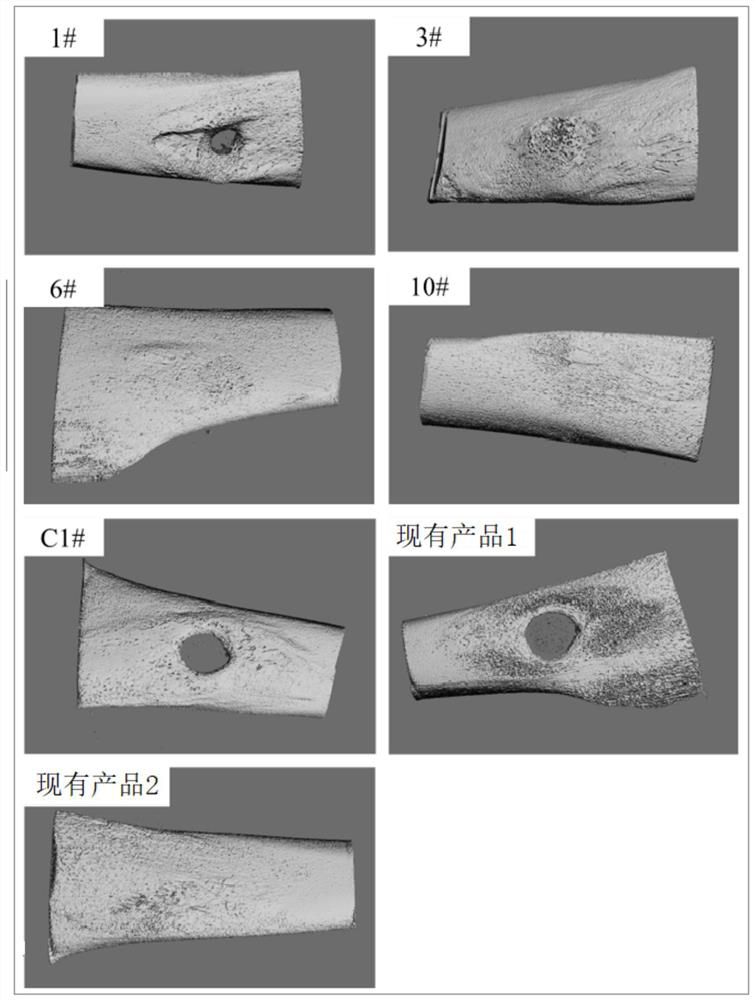
Image
Examples
Embodiment 1
[0060] This embodiment discloses the preparation method of the amphiphilic block copolymer of the present invention, specifically:
[0061] Weigh 10.2 g of the dried polyethylene glycol 1000, 29.4 g of trimethylene carbonate, and 14.0 g of ε-caprolactone, add them into a 250 mL three-necked bottle, and then add 3 mg of stannous octoate as a catalyst. Under the protection of nitrogen, raise the temperature to 150°C, stir and react for 12 hours, then add ε-caprolactone 46.5g, continue to stir and react for 36 hours, cool to room temperature, purify and dry to obtain an amphiphilic block copolymer as 1# bone wax.
Embodiment 2
[0063] This embodiment discloses the preparation method of the amphiphilic block copolymer of the present invention, specifically:
[0064] Weigh 8.6 g of the dried polyethylene glycol monomethyl ether 600, 16.8 g of trimethylene carbonate, and 46.6 g of ε-caprolactone, add them into a 250 mL three-necked bottle, and then add 3 mg of stannous octoate as a catalyst. Under the protection of nitrogen, raise the temperature to 150°C, stir and react for 24 hours, then add ε-caprolactone 28.0g, continue to stir and react for 48 hours, cool to room temperature, purify and dry to obtain an amphiphilic block copolymer as 2# bone wax.
Embodiment 3
[0066] This embodiment discloses the preparation method of bone wax of the present invention, specifically:
[0067] Weigh 86g of 1# bone wax and 14g of four-armed polyethylene glycol 10000, add it into a 250mL three-necked bottle, heat up to 80°C until the materials are completely melted, stir and blend for 2 hours, and cool to room temperature to obtain 3# bone wax.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com