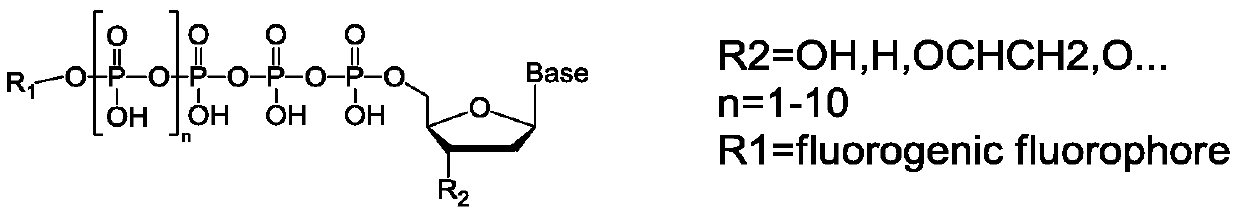
Method for synthesizing anthracene fluorescent dye
A technology for fluorescent dyes and anthracenes, which is applied in the field of biochemical synthesis, can solve the problems of difficult preparation of key intermediates, limited reaction conditions, and low synthesis yields, avoiding the use of organometallic compounds, simple preparation, and cheap and readily available raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
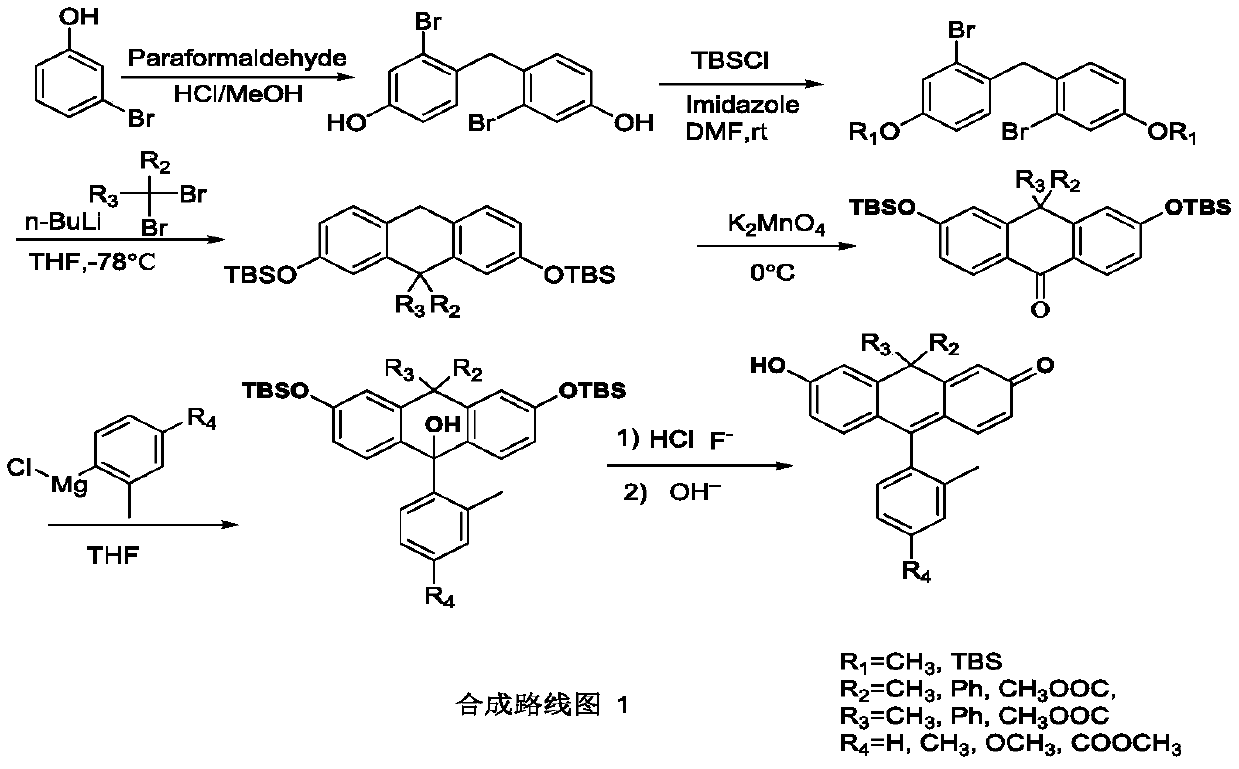
[0057] The invention discloses a method for synthesizing anthracene compounds, which is characterized in that it comprises:
[0058] (1) Take m-halogenated phenol or substituted m-halogenated phenol; dissolve in methanol aqueous solution of concentrated hydrochloric acid; add paraformaldehyde, heat to 40-60 degrees Celsius, and react for 10-40 hours; separate the product to obtain diphenylmethane Derivatives; i.e. first intermediates;
[0059] (2) reacting the first intermediate product with tert-butyldimethylchlorosilane to obtain a diphenylmethane derivative with a phenolic hydroxyl protecting group TBS; the second intermediate product;
[0060] (3) reacting the second intermediate product with n-butyllithium to obtain an organic lithium salt, and reacting it with the dihalogenated compound 2,2-dibromopropane to obtain an anthracene derivative, that is, the third intermediate product;
[0061] (4) oxidizing the third intermediate product with potassium permanganate to obtai...
Embodiment 1
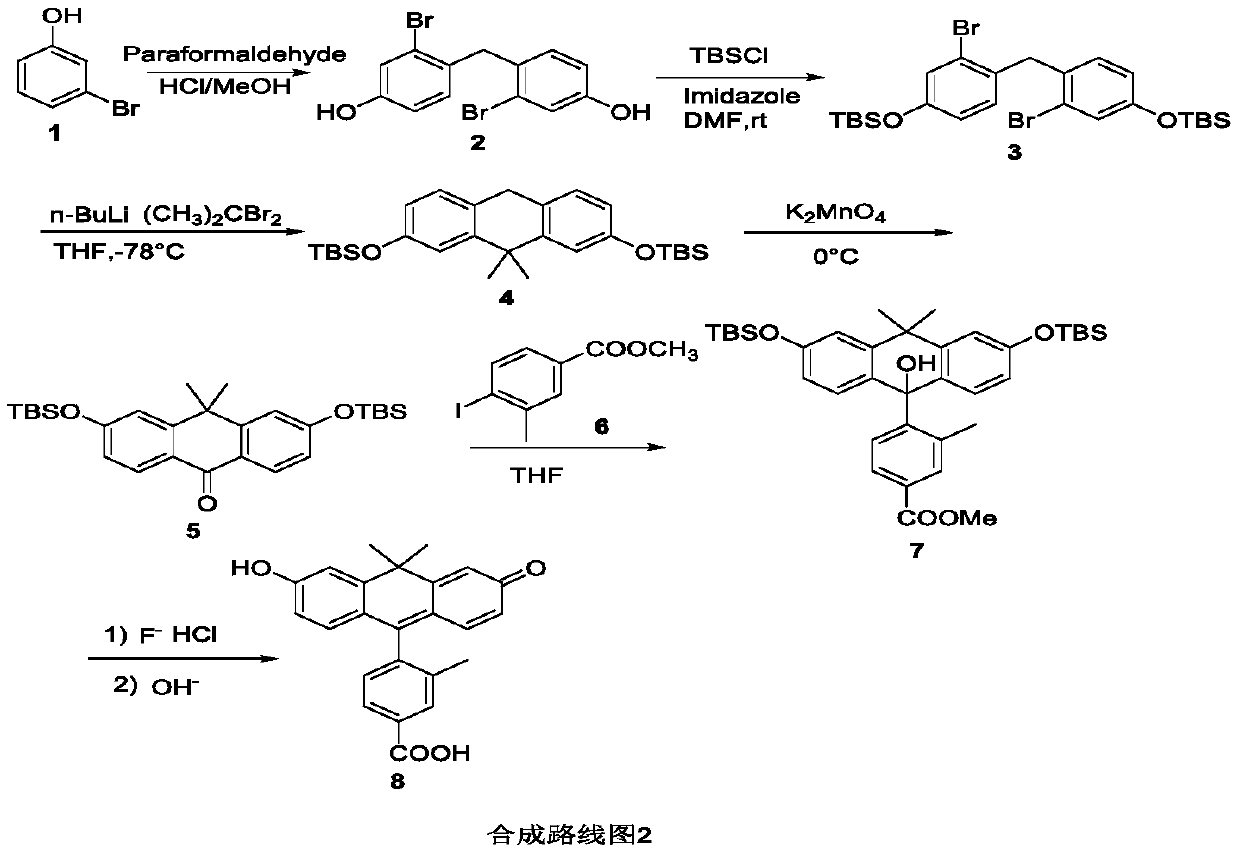
[0067] The synthesis of COOH-PO fluorescent dye is shown in Synthetic Route 2;
[0068]
[0069] 1) Synthesis of compound 4,4'-methylenebis(3-bromophenol)2: weigh compound 1 (10g, 57.8mmol), dissolve in 40% methanol and water (1:1) solution of concentrated hydrochloric acid, add polymer Formaldehyde (360 mg, 4 mmol) was used to heat the reaction solution to 50°C for 24 hours. After most of the methanol in the water was spun off from the reaction solution, brine was added, extracted with dichloromethane, and the solvent was spun off and purified by column chromatography to obtain 3.6 g of oily compound 2.
[0070] 1H NMR (300MHz, CDCl3) δ8.95(s, 2H), 7.22(dt, J=7.5, 1.1Hz, 2H), 7.01(d, J=1.5Hz, 2H), 6.75(dd, J=7.5, 1.5Hz,2H),4.02(t, J=1.0Hz,2H).13C NMR(75MHz,CDCl3)δ156.32,131.75,127.53,125.48,118.98,118.58,42.17.HRMS:Caled for C13H11Br2O2(M+H), 358.0290, Found m / z 358.0291.
[0071]
[0072] 2) Synthesis of compound bis(2-bromo-4-((tert-butyldimethylsilyl)oxy)phenyl)me...
specific Embodiment example 2
[0091] The synthesis of COOH-TM fluorescent dye is shown in Synthetic Route 3
[0092]
[0093] 1 compound 4,4'-methylenebis (3-bromophenol) 2 synthesis:
[0094] Weigh compound 1 (10g, 57.8mmol), dissolve in methanol and water (1:1) solution of 40% concentrated hydrochloric acid, react at room temperature for 20 minutes, add paraformaldehyde (360mg, 4mmol) and heat the reaction solution to 50 °C, react for 24 hours. After most of the methanol in the water was spun off from the reaction solution, brine was added, extracted with dichloromethane, and the solvent was spun off and purified by column chromatography to obtain 3.6 g of oily compound 2.
[0095] 1H NMR (300MHz, CDCl3) δ8.95(s, 2H), 7.22(dt, J=7.5, 1.1Hz, 2H), 7.01(d, J=1.5Hz, 2H), 6.75(dd, J=7.5, 1.5Hz,2H),4.02(t, J=1.0Hz,2H).13C NMR(75MHz,CDCl3)δ156.32,131.75,127.53,125.48,118.98,118.58,42.17.HRMS:Caled for C13H11Br2O2(M+H), 358.0290, Found m / z 358.0291.
[0096]
[0097] 2 Compound bis(2-bromo-4-((tert-but...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com