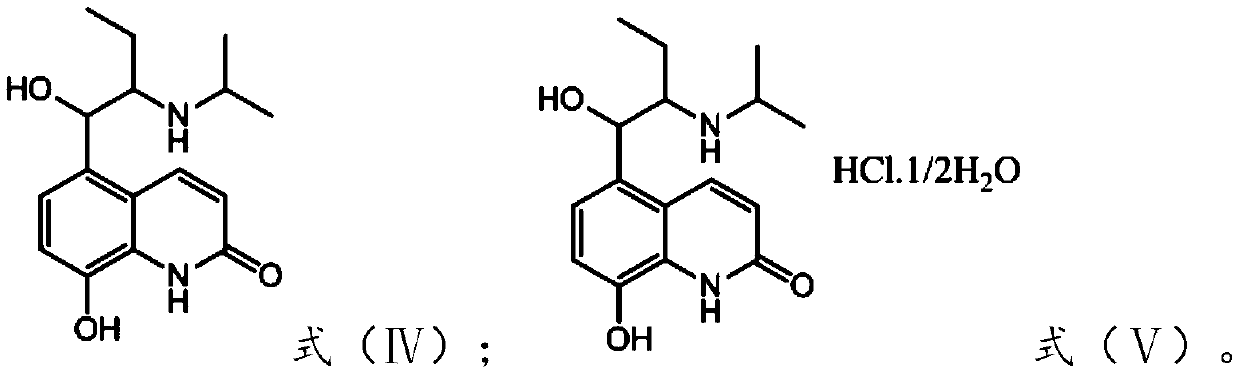
Preparation method of procaterol hydrochloride
A technology of procaterol hydrochloride and procaterol, which is applied in the direction of organic chemistry, can solve the problems of high production cost, achieve the effects of low price, save preparation time, and improve preparation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
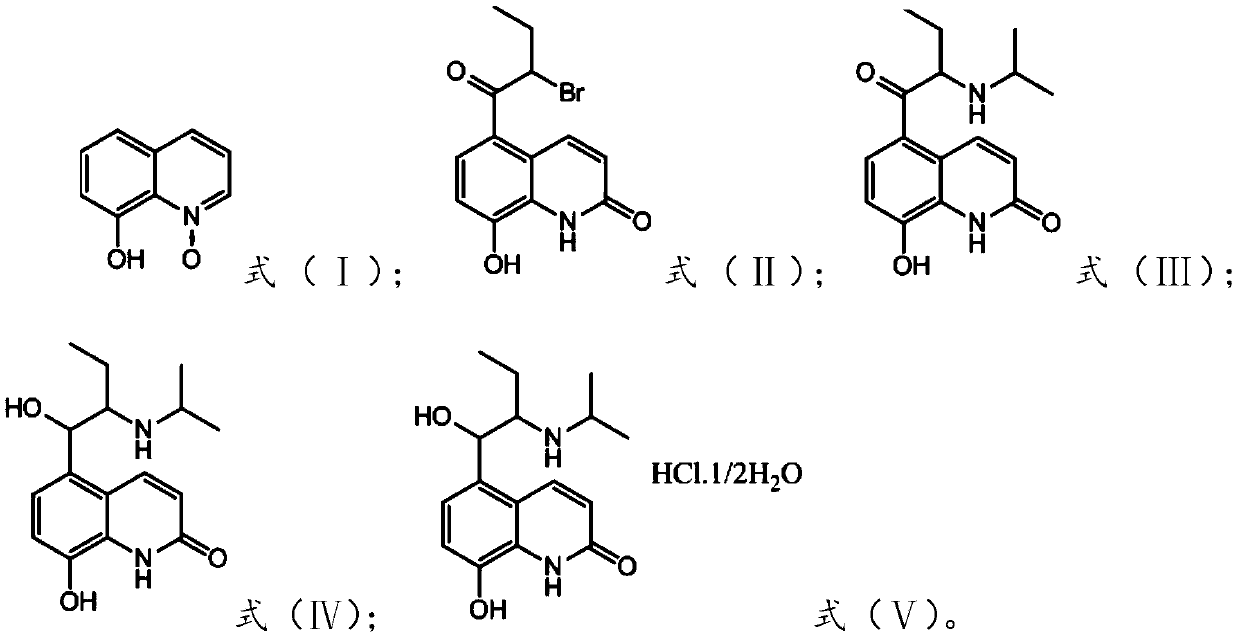
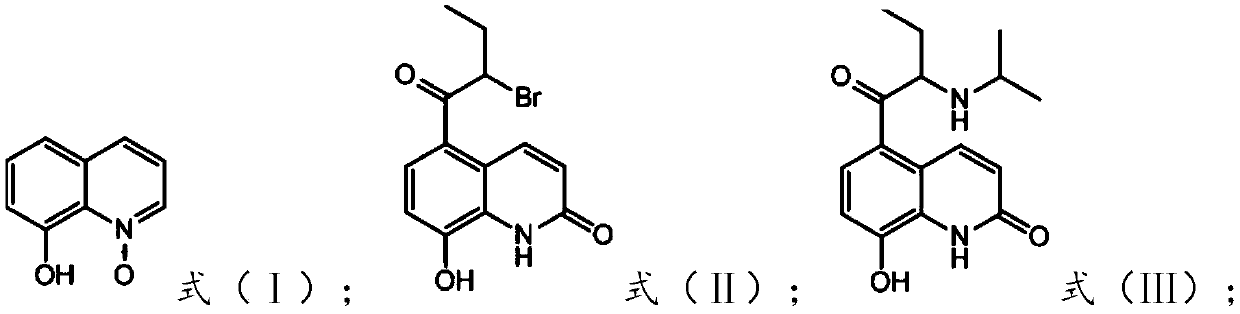
[0049] The preparation of the present embodiment 5-(2-bromobutyryl)-8-hydroxyquinolone
[0050] Add carbon disulfide (600mL), 8-hydroxyquinoline nitrogen oxide (79.59g, 0.5mol), 2-bromobutyryl bromide (344.85g, 1.50mol) and aluminum trichloride ( 133.34g, 1.00mol), reacted for 3 hours at 25°C, filtered, recrystallized and dried with 800ml 95% ethanol to obtain yellow crystal 5-(2-bromobutyryl)-8-hydroxyquinolone (100.8g), yield 78%, Its purity was greater than 98% by HPLC.
[0051] The detection result of 5-(2-bromobutyryl)-8-hydroxyquinolone proton NMR spectrum is: 1 HNMRδ: 7.93~7.96(d, J=12Hz, 1H, ArH), 7.72~7.74(d, J=8Hz, 1H, ArH), 7.27~7.30(d, J=12Hz, 1H, ArH), 6.71~6.73 (d, J=8Hz, 1H, ArH), 11.27(s, 1H, NH), 12.28(s, 1H, OH), 5.70~5.74(t, J=8Hz, 1H, CH), 1.95~2.20(m ,2H,CH2 ), 1.02~1.06(t, J=8Hz, 3H, CH 3 )
Embodiment 2
[0053] This embodiment is the preparation of 5-(2-bromobutyryl)-8-hydroxyquinolone
[0054] Add nitrobenzene (550mL), 8-hydroxyquinoline nitrogen oxide (79.59g, 0.5mol), 2-bromobutyryl chloride (278.18g, 1.50mol) and aluminum trichloride to a 1000mL there-necked flask with mechanical stirring (133.34g, 1.00mol), reacted at 70°C for 6 hours, filtered, recrystallized from 700ml 95% ethanol and dried to obtain yellow crystal 5-(2-bromobutyryl)-8-hydroxyquinolone (96.15g), yield 75% , and its purity was greater than 98% as detected by HPLC.
Embodiment 3
[0056] Preparation of 5-(2-methylisopropylaminobutyryl)-8-hydroxyquinolone
[0057] Add methanol (600mL), 5-(2-bromobutyryl)-8-hydroxyquinolone (92.97g, 0.3mol) prepared in Example 1, isopropylamine (26.60g, 0.45 mol), be warming up to 60 DEG C and react for 8 hours, be down to room temperature, the pH of the reaction solution is adjusted to 2 with hydrochloric acid, and the solid is precipitated, filtered and washed with water to obtain 5-(2-methylisopropylaminobutyryl)-8-hydroxyquinolone ( 77.85g), its purity was greater than 99% as detected by HPLC, and its yield was 90%.
[0058] The detection result of 5-(2-methylisopropylamine butyryl)-8-hydroxyquinolone proton NMR spectrum is: 1 HNMRδ: 8.24~8.21(d, J=12Hz, 1H, ArH), 7.18~7.16(d, J=8Hz, 1H, ArH), 6.95~6.93(d, J=8Hz, 1H, ArH), 6.79~6.77 (d,J=8Hz,1H,ArH),11.43(s,1H,NH),4.13~4.11(m,1H,CH),3.98~3.66(q,J=4Hz,1H,CH),1.58~1.75 (m,2H,CH 2 ), 1.51~1.54 (t, J=8Hz, 6H, CH 3 ),0.71~0.74(t,J=8Hz,3H,CH 3 )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com