Five-membered ring phosphite compound, and preparation method and application thereof
A kind of cyclic phosphite, compound technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
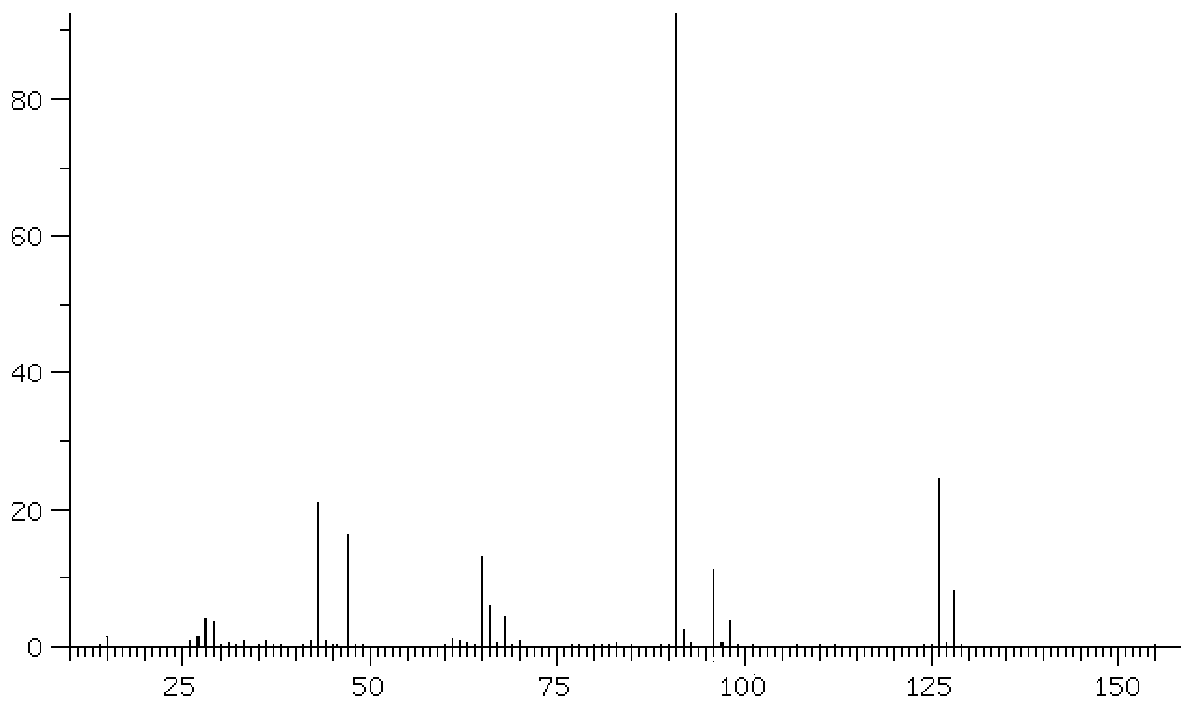
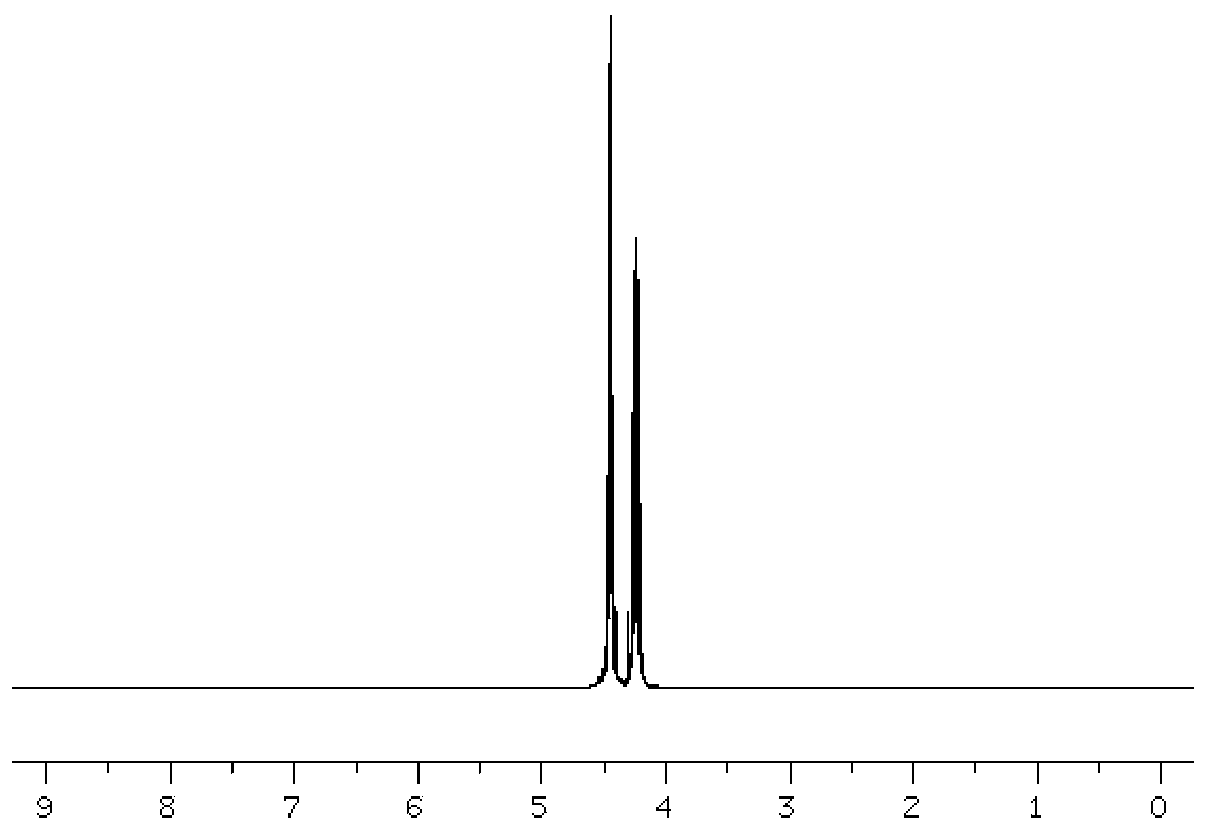
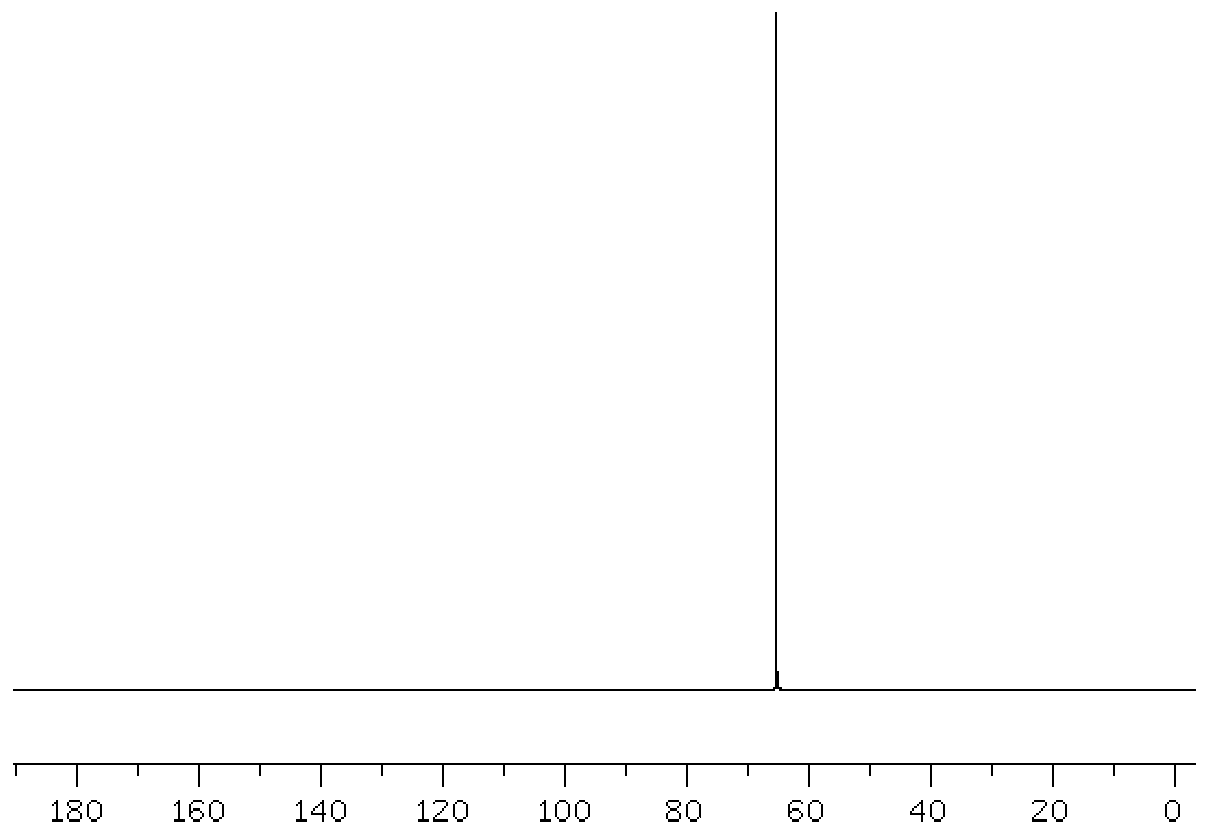
Image
Examples
preparation example Construction
[0047] The embodiment of the present invention also provides a method for preparing a five-membered ring phosphite compound, comprising the following steps:
[0048] S10. Dissolving compound A in a non-aqueous solvent, adding phosphorus trichloride to react, and separating to obtain compound B;
[0049] S20. After mixing the compound B and compound C, react at a temperature of -40°C to 60°C, and separate and obtain five-membered ring phosphite compounds;
[0050] Wherein, the reactant A is selected from: and / or The reactant C is selected from: and / or
[0051] Among them, R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R 9 , R 10 , R 11 , R 12 , R 13 , R 14 , R 15 , R 16 , R 17 and R 18 are independently selected from: hydrogen, alkyl, alkenyl, alkynyl, alkoxy, alkynyloxy, alkenyloxy, silyl, siloxane, arylsilyl, arylsiloxy, haloalkyl , phenyl, biphenyl, naphthyl, pyridyl, thienyl, halogenated phenyl, halogenated biphenyl, phenol, alkyl-containing ...
Embodiment 1
[0092] In order to be able to compare the preparation process of the more intuitive comparison products in parallel, the same self-made compound B (that is, the compound B prepared in Example 1) was used in Examples 2 to 33 of the present invention. The specific preparation process includes:
[0093] ①At room temperature, add 310g of ethylene glycol (reactant A) and 350g of anhydrous dichloromethane (non-aqueous solvent) into a three-neck flask with a volume of 2000mL in a fume hood, then cool down to 0°C and continue stirring for 0.5h. At this time, the solution is colorless and transparent, and the three-necked bottle is connected to an external gas absorption device.
[0094] ② Slowly add 700g of phosphorus trichloride dissolved in 300g of anhydrous dichloromethane into the three-necked flask through the dropping funnel, and control the dropping rate to 1 drop / second. With the continuous dropwise addition of the dichloromethane solution of phosphorus trichloride, the reacti...
Embodiment 2
[0098] Compound 1 ( Preparation of two (1,3,2-dioxaphospholane) glycol esters):
[0099] Add 400g of dichloromethane (solvent, the same effect as the aforementioned non-aqueous solvent, optional use) and 62g of ethylene glycol (reactant C) into a 1000mL three-necked flask at room temperature, the solution is colorless and transparent, and the three-necked flask is connected Connect an external gas absorption device, then slowly add 300g of compound B prepared in Example 1 into a 1000mL three-necked flask through a dropping funnel, control the dropping rate at 1 drop / second, and the reaction temperature at 0°C. With the dropwise addition of compound B, A large amount of heat is released and a large amount of HCl gas is released. After all the drops are completed, the solution is colorless and transparent (dropping compound B to this point for 1.5h), and then the reaction is slowly raised to room temperature and continued to stir for 12h. The light yellow solid was obtained fr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Radius | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com