Method for synthesizing Vibativ intermediate
A synthesis method and telavancin technology, applied in the field of medicine, can solve problems such as high cost, and achieve the effects of reduced synthesis cost, less side reactions, and easy control of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
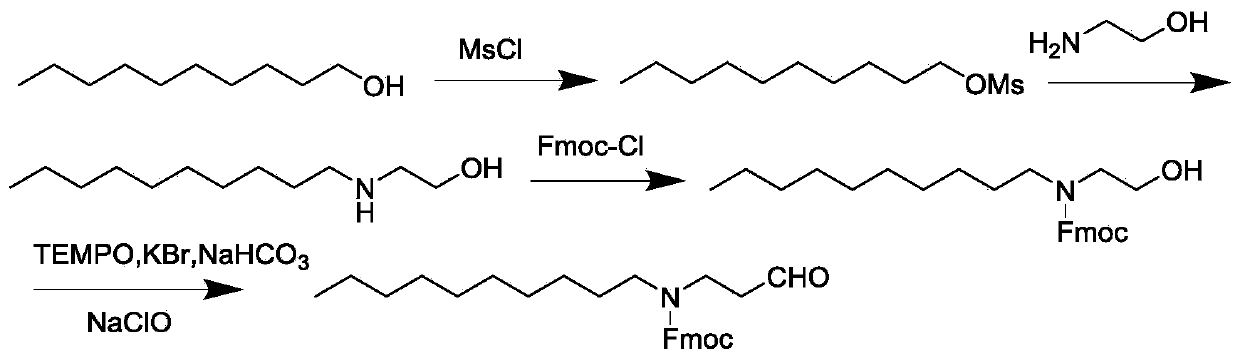
[0026] Such as figure 1 Shown, a kind of telavancin intermediate synthetic method of the present embodiment, the purpose is to provide the method for synthesizing telavancin intermediate N-(9-fluorenylmethoxycarbonyl) decyl amino acetaldehyde: with n-decyl Alcohol and aminoethanol are starting materials, through condensation, Fmoc-Cl amino protection, oxidation to obtain N-(9-fluorenylmethoxycarbonyl)decylaminoacetaldehyde, the present invention selects 2,2,6,6-tetra Methyl piperidine oxide (TEMPO) is an oxidizing agent, the reaction temperature is below 0°C, and dichloromethane is a solvent to selectively control the oxidation of primary alcohols into aldehydes, including the following steps:
[0027] (1) At room temperature, dissolve n-decyl alcohol with dichloromethane, add organic base 1, add dropwise methanesulfonyl chloride, and react for 12 hours;
[0028] (2) adding the reaction solution after reacting for 12 hours into ice water, layering, and concentrating to obtain...
Embodiment 1
[0039] The synthesis of embodiment 1N-n-decylaminoethanol:
[0040] Take 8g (50mmol) of n-decyl alcohol and add it to a three-necked flask, stir and dilute with 25ml of dichloromethane, add 4g (59mmol) of triethylamine, control the reaction temperature at 0°C, add 8g (70mmol) of methanesulfonyl chloride dropwise, and the dropwise addition is completed. Warm up to room temperature and react for 12h. TLC (iodine fumigation) detected the completion of the reaction, added ice water, separated layers, collected the organic layer, dried over sodium sulfate, and concentrated to obtain concentrated solution 1, which can be carried out in the next step without purification. Concentrate 1 was dissolved in 30ml of absolute ethanol, transferred to a three-necked flask, added with 30g (0.5mol) of aminoethanol, slowly raised to 90°C, and reacted for 12h. Add 100ml dichloromethane and 100ml water, separate layers, the organic layer is dried over sodium sulfate, concentrates, obtains concent...
Embodiment 2
[0041] The synthesis of embodiment 2N-n-decylaminoethanol:
[0042] Take 8g (50mmol) of n-decyl alcohol and add it to a three-necked flask, stir and dilute with 25ml of dichloromethane, add 4g (59mmol) of triethylamine, control the reaction temperature at 0°C, add 8g (70mmol) of methanesulfonyl chloride dropwise, and the dropwise addition is completed. Warm up to room temperature and react for 12h. TLC (iodine fumigation) detected the completion of the reaction, added ice water, separated layers, collected the organic layer, dried over sodium sulfate, and concentrated to obtain concentrated solution 1, which can be carried out in the next step without purification. Concentrate 1 was dissolved in 30ml of absolute ethanol, transferred to a three-necked flask, 60g (1mol) of aminoethanol was added, the temperature was slowly raised to 90°C, and reacted for 12h. Add 100ml dichloromethane and 100ml water, layering is difficult, do not carry out next step reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com