Preparation method of cyclic carbonate
A cyclic carbonate and carbon atom technology, applied in the field of preparation of cyclic carbonate, can solve the problems of poor selectivity, easy hydrolysis, oxidation, etc., and achieve the effects of no metal residue, improved reaction efficiency, and improved activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Catalyst Synthesis:
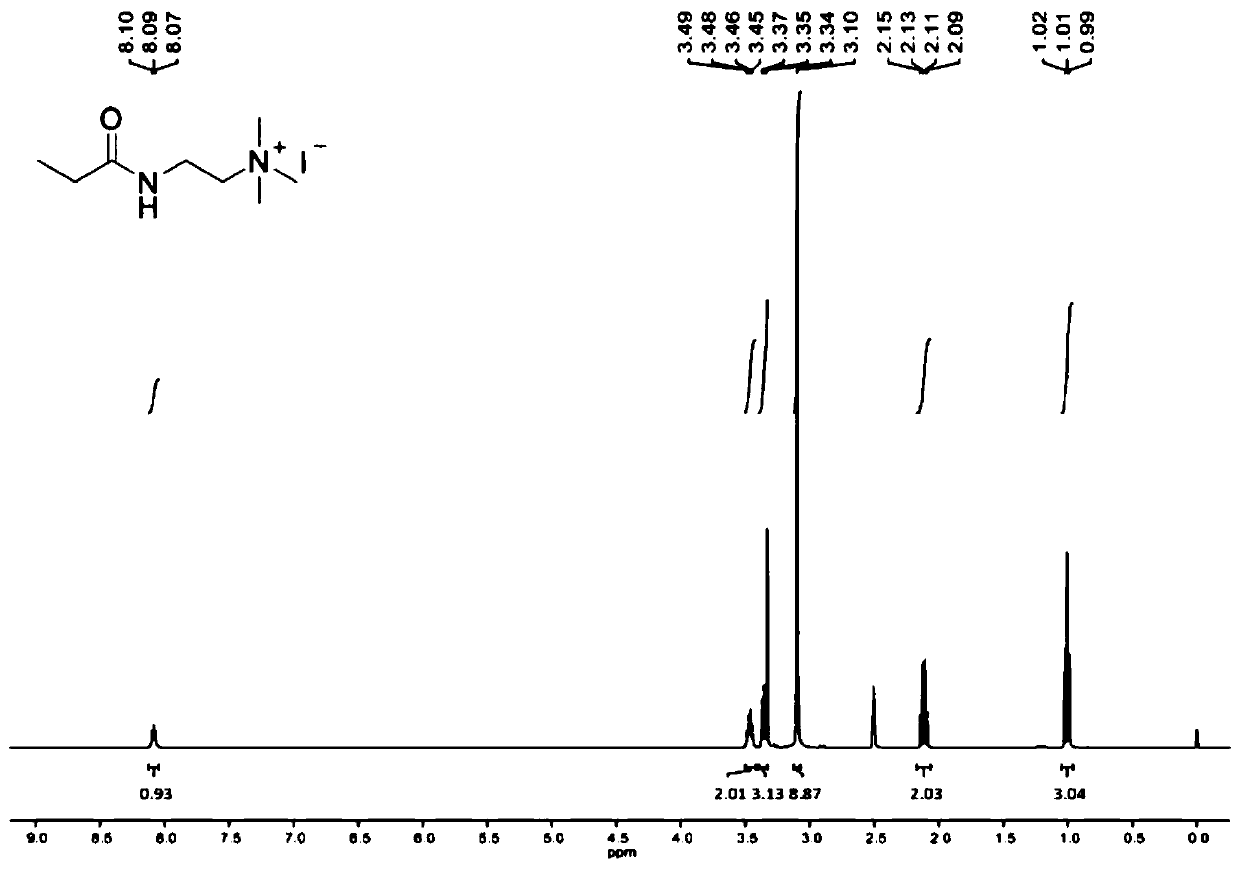
[0069] Dissolve triethylamine (3.0mL, 21.97mmol, 1.2equiv) in dichloromethane (40mL), cool to 0°C, add N,N-dimethylethylenediamine (2.0mL, 18.31mmol, 1.0equiv) . Propionyl chloride (1.6 mL, 18.31 mmol, 1.0 equiv) was added dropwise using a syringe, and the addition was completed in about 20 min. The reaction solution was warmed to room temperature, stirred overnight, and saturated NaHCO 3 The reaction was quenched with aqueous solution with CH 2 Cl 2 Extract 3 times. An appropriate amount of anhydrous magnesium sulfate dried the organic layer, concentrated in vacuo, and separated by chromatography (silica gel, 10% MeOH / CH 2 Cl 2 ) to obtain the product as a brownish-yellow viscous liquid, add 20ml of acetone to dissolve, add excess methyl iodide dropwise, under room temperature, after continuous stirring for 2h, there is precipitation, suction filtration, recrystallization, vacuum drying (50 ° C, 24h) , the product Catalyst 1 was obtained as...
Embodiment 2
[0074] Catalyst Synthesis:
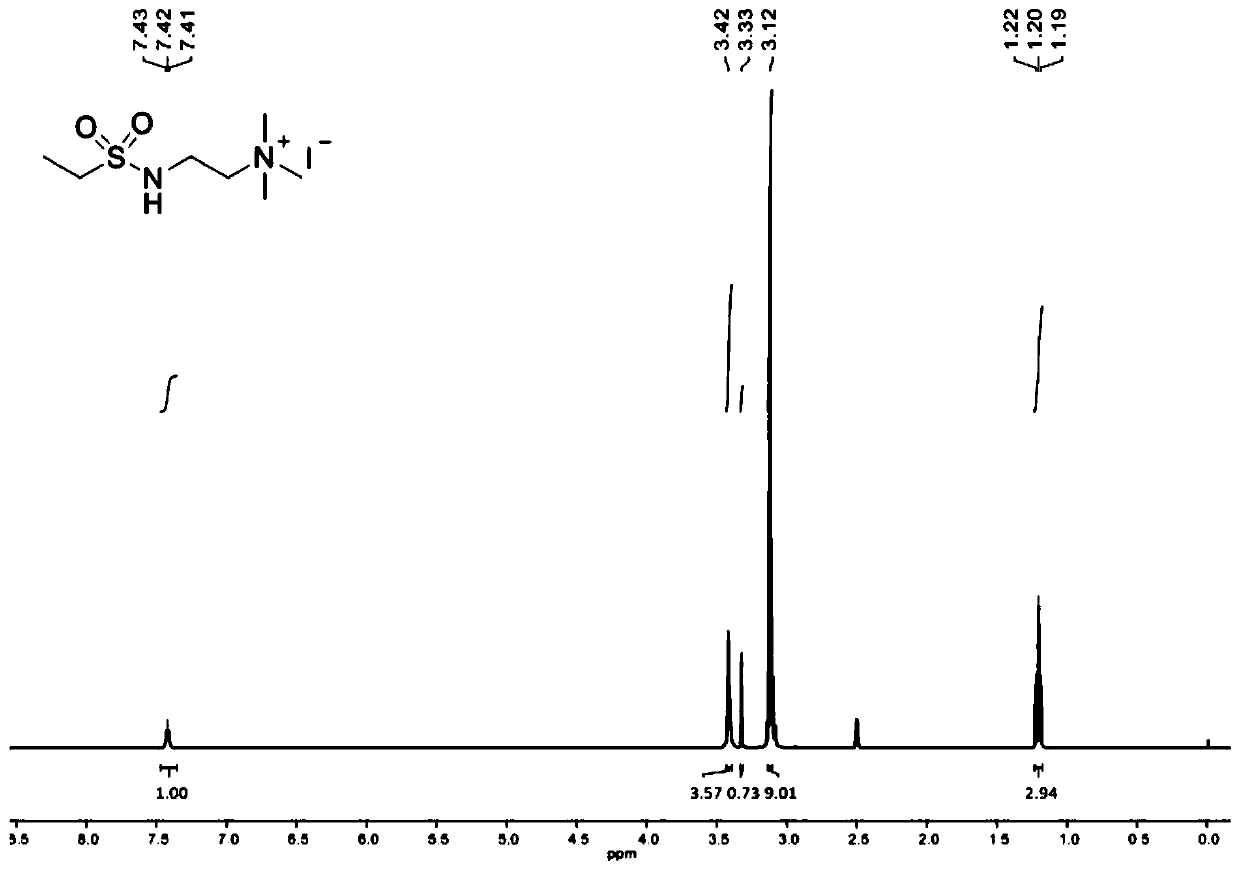
[0075] Dissolve triethylamine (3.0mL, 21.97mmol, 1.2equiv) in dichloromethane (40mL), cool to 0°C, add N,N-diethylethylenediamine (2.6mL, 18.31mmol, 1.0equiv) . tert-Butylsulfinyl chloride (2.3 mL, 18.31 mmol, 1.0 equiv)) was added dropwise using a syringe, and the addition was completed in about 20 min. The reaction solution was warmed to room temperature, stirred overnight, and saturated NaHCO 3 The reaction was quenched with aqueous solution with CH 2 Cl 2 Extract 3 times. An appropriate amount of anhydrous magnesium sulfate dried the organic layer, concentrated in vacuo, and separated by chromatography (silica gel, 10% MeOH / CH 2 Cl 2 ) to obtain the product as a brownish-yellow viscous liquid, add 20ml acetone to dissolve, add excess ethyl chloride dropwise, under room temperature, after continuous stirring for 6h, there is precipitation, suction filtration, recrystallization, vacuum drying (50 ℃, 24h ), the product Catalyst 2 was obtain...
Embodiment 3
[0079] Catalyst Synthesis:
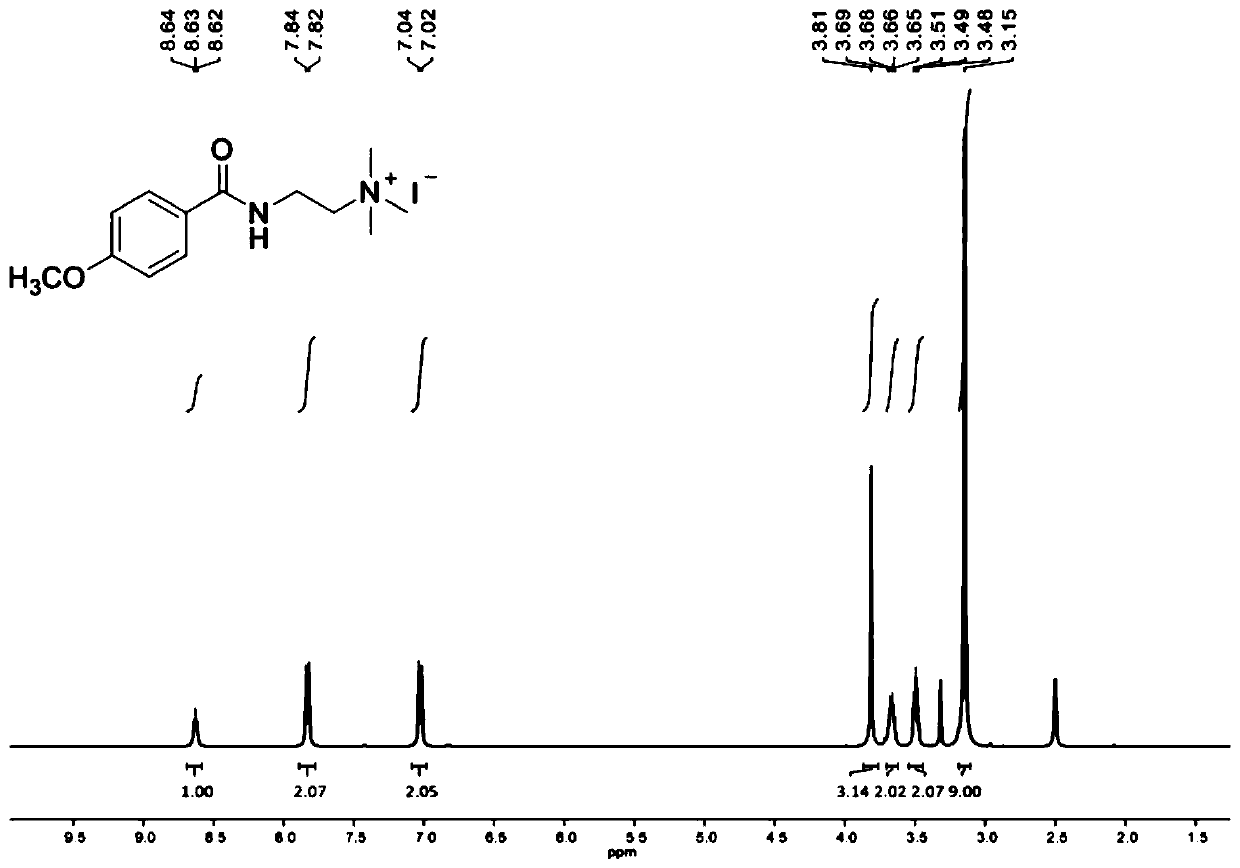
[0080] Dissolve triethylamine (3.0mL, 21.97mmol, 1.2equiv) in dichloromethane (40mL), cool to 0°C, add N,N-dimethylethylenediamine (2.0mL, 18.31mmol, 1.0equiv) . Trifluoromethanesulfonyl chloride (1.9 mL, 18.31 mmol, 1.0 equiv) was added dropwise using a syringe, and the addition was completed in about 20 min. The reaction solution was warmed to room temperature, stirred overnight, and saturated NaHCO 3 The reaction was quenched with aqueous solution with CH 2 Cl 2 Extract 3 times. An appropriate amount of anhydrous magnesium sulfate dried the organic layer, concentrated in vacuo, and separated by chromatography (silica gel, 10% MeOH / CH 2 Cl 2) to obtain the product as a brownish-yellow viscous liquid, add 20ml of acetone to dissolve, add excess methyl bromide dropwise, under room temperature, after continuous stirring for 2h, there is precipitation, suction filtration, recrystallization, vacuum drying (50 ° C, 24h), The product catalyst 3 w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com