Method for analyzing stable hydrogen isotope ratio of non-exchangeable hydrogen in food total sugar
A hydrogen isotope and ratio technology, applied in the field of stable isotope technology research, can solve problems that hinder research and application, complicated operation, etc., and achieve the effects of rapid analysis, high efficiency, low experimental cost, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
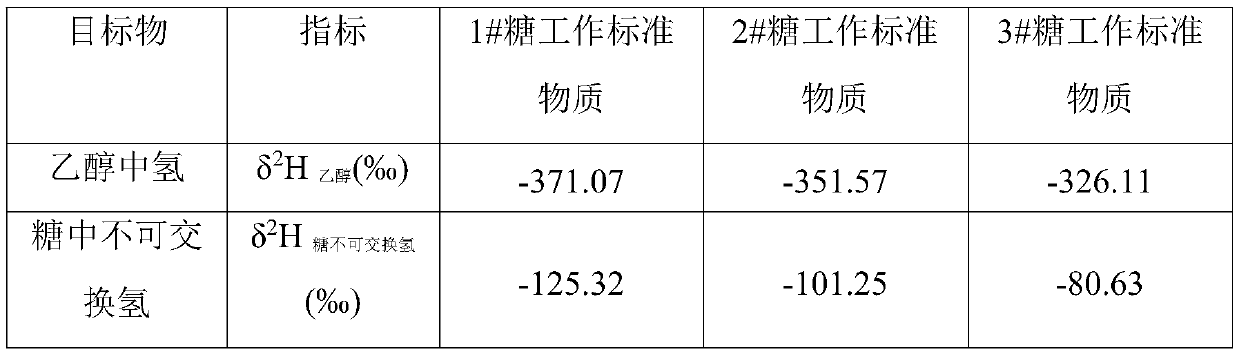
[0029] Example 1 Establishment of the δ of ethanol in fermentation broths of sugar compounds as a working standard 2 h 乙醇 Non-exchangeable hydrogen δ with sugar 2 h 糖不可交换氢 relational model
[0030] 1) Select three sugar compound working standard substances, whose δ of non-exchangeable hydrogen 2 h 糖不可交换氢 The values are -125.35‰, -101.25‰ and -80.63‰ respectively; among them, the working standard sugar compound is preferably glucose, whose δ of non-exchangeable hydrogen 2 h 糖不可交换氢 Values were determined by the method proposed by John Dunbar et al.
[0031] 2) Three sugar compounds used as working standards were respectively prepared into 170g / L sugar aqueous solution, after adding 0.5g active dry yeast, alcoholic fermentation was carried out at 30±0.5°C;
[0032] 3) Take the clarified liquid after the centrifugation of the fermented liquid, and measure the δ of ethanol in the fermented liquid with a gas chromatography-cracking-stable isotope ratio mass spectrometer ...
Embodiment 2
[0036] Example 2 Analysis of hydrogen isotope ratios of total sugar non-exchangeable hydrogen in acacia honey
[0037]Take a kind of acacia honey as the research object, remove the moisture in the honey by freeze-drying, and then prepare the solid powder into a 170g / L sugar aqueous solution, add 0.5g active dry yeast to the 100g solution sample for alcohol fermentation, and determine δ of ethanol in fermentation broth 2 h 乙醇测定 This process was repeated three times, and the results are shown in Table 2.
[0038] Table 2 δ of ethanol fermented by acacia honey samples 2 h 乙醇测定 Value repeatability determination results
[0039] index repeat -1 Repeat -2 repeat -3 δ 2 h 乙醇测定 (‰)
-315.88 -311.26 -310.33
[0040] The standard deviation of the results of three repeated analyzes was 2.97‰, which met the requirements of stable isotope analysis (better than 3‰).
[0041] Substitute the data in Table 2 into the relational model of Example 1, and c...
Embodiment 3
[0044] Example 3 Analysis of hydrogen isotope ratios of non-exchangeable hydrogen of total sugars in linden honey
[0045] Take 3 linden honey as the research object, remove the water in the honey by freeze-drying, and then prepare the solid powder into 170g / L sugar aqueous solution, add 0.5g active dry yeast to 100g solution sample for alcoholic fermentation , to determine the δ of ethanol in the fermentation broth 2 h 乙醇测定 values, and the results are shown in Table 4.
[0046] Table 4 δ of ethanol converted from linden honey 2 h 乙醇测定 The measurement results
[0047] index Linden Honey-1# Linden honey-2# Linden honey-3# δ 2 h 乙醇测定 (‰)
[0048] Substitute the data in Table 4 into the relational model obtained in Example 1, and calculate the δ of the non-exchangeable hydrogen of sugar 2 h 糖不可交换氢计算 values, and the results are shown in Table 5.
[0049] Table 5 δ of non-exchangeable hydrogen of sugar in Linden honey 2 h 糖不可交换氢计算 value calculati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com