Polyvinyl imidazole functionalized polysulfone microfiltration membrane as well as preparation method and application thereof
A technology of polyvinylimidazole and vinylimidazole, which is applied in the field of polymer membrane materials, can solve the problems of low ion filtration efficiency and achieve good chemical stability, easy operation, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
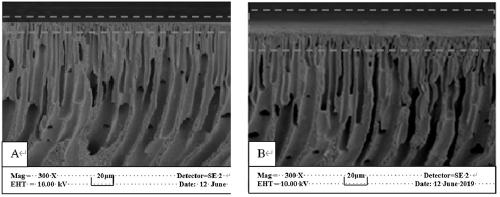
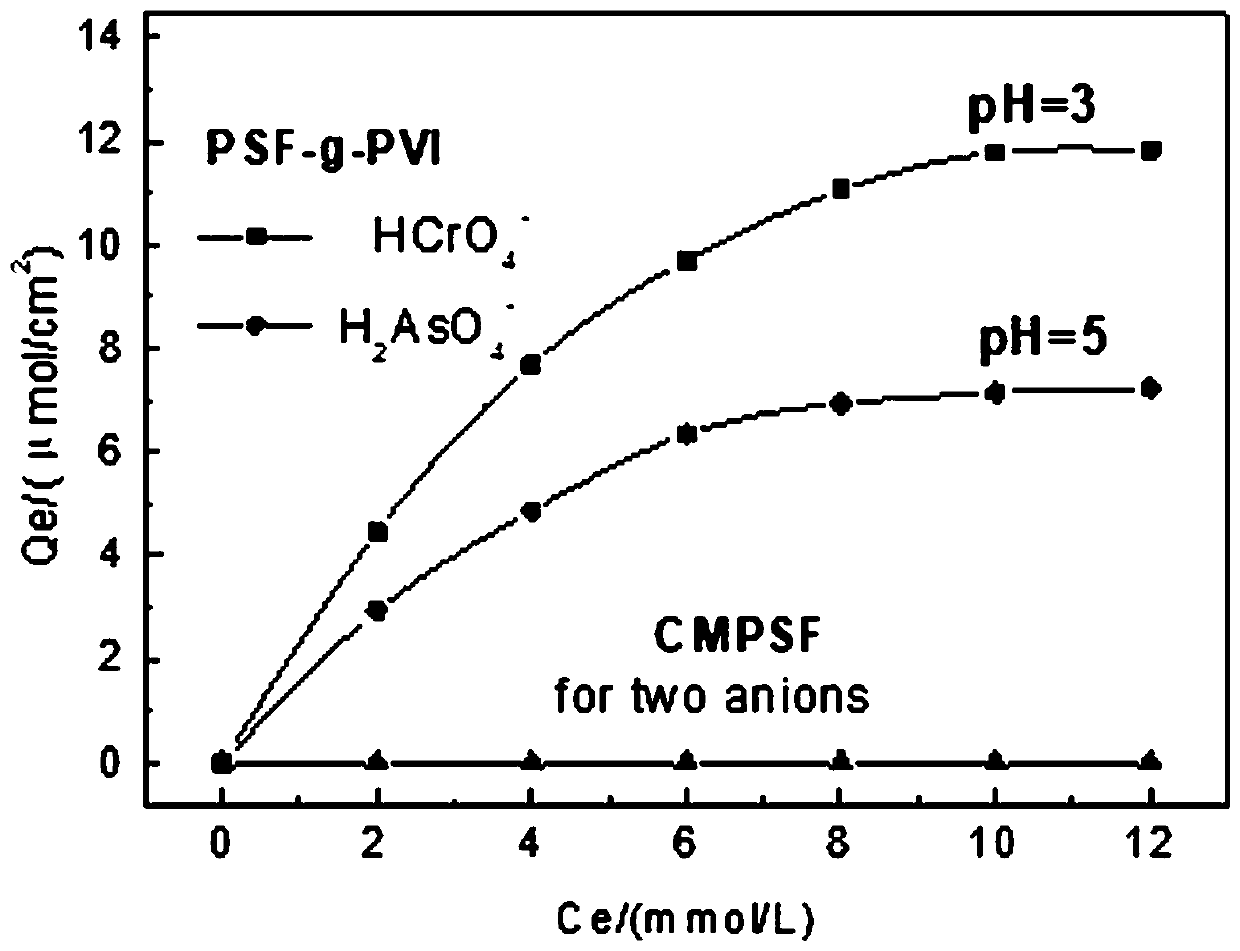
[0032] In a four-neck flask equipped with a stirrer, a condenser, and a thermometer, add 0.1g CMPSF and 60mL of a mixed solvent composed of ethanol, water, and DMF (volume ratio = 1:1:2), and after swelling for 2 hours, add 0.13 g of 3-hydroxy-N,N-diethylaniline (HDEA) and 0.12 g Na 2 CO 3 , N 2 Protected, stirred at 80°C for 6 h, the membrane was taken out, washed repeatedly with a mixed solvent of ethanol and distilled water at a volume ratio of 1:1, and dried in vacuum to constant weight to obtain a modified polyamide with a side chain bonded to an aromatic tertiary amine. Sulfone membrane PSF-DEA, the binding amount is 1.72umol / cm 2 .
[0033] Add 0.1 g PSF-DEA membrane to 70 mL DMF, after swelling for 2 hours, add 3.2 mL monomer vinylimidazole (VI), N 2 After bubbling for half an hour, raise the temperature to 70°C, add 0.04 g initiator, react for 10 h, take out the membrane, soak it in DMF and ethanol and wash the grafted membrane repeatedly, and dry it in vacuum to ...
Embodiment 2
[0035] In a four-neck flask equipped with a stirrer, a condenser, and a thermometer, add 0.1g CMPSF and 60mL of a mixed solvent composed of ethanol, water, and DMF (volume ratio = 1:1:2), and after swelling for 2 hours, add 0.13 g of 3-hydroxy-N,N-diethylaniline (HDEA) and 0.125 g NaHCO 3 , N 2 protected, stirred at 70°C for 7 h, the membrane was taken out, washed repeatedly with a mixed solvent of ethanol and distilled water at a volume ratio of 1:1, and dried in vacuo to constant weight to obtain a modified polyamide with a side chain bonded to an aromatic tertiary amine. Sulfone membrane PSF-DEA, the binding amount is 1.64umol / cm 2 .
[0036] Add 0.1 g PSF-DEA membrane to 70 mL DMF, after swelling for 2.5 hours, add 3.1 mL monomer vinylimidazole (VI), N 2 After bubbling for half an hour, raise the temperature to 70°C, add 0.05 g initiator, react for 11 h, take out the membrane, soak it in DMF and ethanol and wash the grafted membrane repeatedly, dry it in vacuum to const...
Embodiment 3
[0038] In a four-neck flask equipped with a stirrer, a condenser, and a thermometer, add 0.1g CMPSF and 60mL of a mixed solvent composed of ethanol, water, and DMF (volume ratio = 1:1:2), and after swelling for 2 hours, add 0.13 g of 3-hydroxy-N,N-diethylaniline (HDEA) and 0.12 g NaOH, N 2 Protected, stirred at 70°C for 6 h, the membrane was taken out, washed repeatedly with a mixed solvent of ethanol and distilled water at a volume ratio of 1:1, and dried in vacuum to constant weight to obtain a modified polyamide with a side chain bonded to an aromatic tertiary amine. Sulfone membrane PSF-DEA, the binding amount is 1 .67umol / cm 2 .
[0039] Add 0.1 g PSF-DEA membrane to 70 mL DMF, after swelling for 3 hours, add 3.3 mL monomer vinylimidazole (VI), N 2 After bubbling for half an hour, the temperature was raised to 65°C, and 0.042 g of initiator was added. After 12 h of reaction, the membrane was taken out, soaked in DMF and ethanol and washed repeatedly, and dried in vacuum...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com