Preparation method of aminobenzonitrile
A technology of aminobenzonitrile and aminobenzamide, which is applied in the field of preparation of aminobenzonitrile, can solve the problems of intractable waste water, low yield, short service life and the like, achieves improved yield and quality, and reduced reaction temperature , the effect of shortened response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
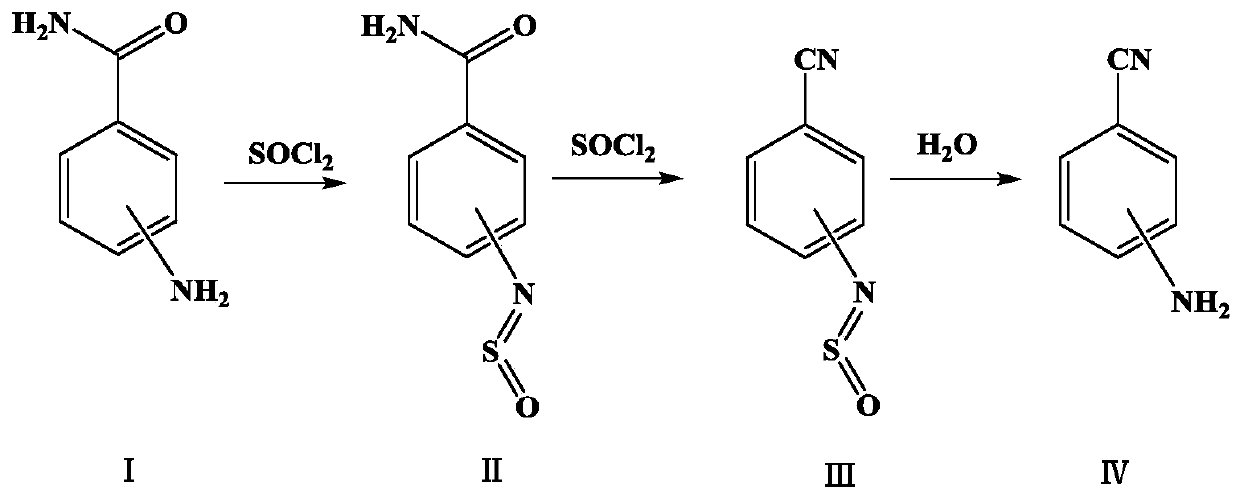
[0022] Dehydration: Add 510 g of toluene and 102 g (0.75 mol) of 4-aminobenzamide into a 1000 ml reaction flask with a reflux device cooled by brine, raise the temperature to 90-100 ° C, slowly add 205 g (1.72 mol) of thionyl chloride dropwise, A large amount of tail gas is produced (HCl+SO 2 ), after the thionyl chloride has been dripped, continue to keep warm until all the materials are dissolved and no tail gas is emitted. Cool to 50-60°C, that is, dehydration liquid, keep warm for later use.
[0023] Hydrolysis: Add 102g of water into a 1000ml reaction bottle, stir and heat up to 50-60°C, add the above dehydration solution dropwise, at this time tail gas will be produced (SO 2 ), control the dropping speed, after the dropping is finished and stirred until no tail gas is released, adjust the pH of the water layer to 6.5-7.5 with 30% sodium hydroxide solution while hot. After standing for separation, the organic layer was slowly stirred and cooled to 0-5°C, filtered, washe...
Embodiment 2
[0025] Dehydration: Add 510g of toluene and 102g (0.75mol) of 3-aminobenzamide into a 1000ml reaction flask with a reflux device cooled by brine, raise the temperature to 90-100°C, slowly add 205g (1.72mol) of thionyl chloride dropwise, A large amount of tail gas is produced (HCl+SO 2 ), after the thionyl chloride has been dripped, continue to keep warm until all the materials are dissolved and no tail gas is released. Cool to 50-60°C, that is, dehydration liquid, keep warm for later use.
[0026] Hydrolysis: Add 102g of water into a 1000ml reaction bottle, stir and heat up to 50-60°C, add the above dehydration solution dropwise, at this time tail gas will be produced (SO 2 ), control the dropping speed, after the dropping is finished and stirred until no tail gas is released, adjust the pH of the water layer to 6.5-7.5 with 30% sodium hydroxide solution while hot. After static separation, the organic layer was slowly stirred and cooled to 0-5°C, filtered, washed with an app...
Embodiment 3
[0028] Dehydration: Add 510g of toluene and 102g (0.75mol) of 2-aminobenzamide into a 1000ml reaction flask with a reflux device cooled by brine, raise the temperature to 90-100°C, slowly add 205g (1.72mol) of thionyl chloride dropwise, A large amount of tail gas is produced (HCl+SO 2 ), after the thionyl chloride has been dripped, continue to keep warm until all the materials are dissolved and no tail gas is released. Cool to 50-60°C, that is, dehydration liquid, keep warm for later use.
[0029] Hydrolysis: Add 102g of water into a 1000ml reaction bottle, stir and heat up to 50-60°C, add the above dehydration solution dropwise, at this time tail gas will be produced (SO 2 ), control the dropping speed, after the dropping is finished and stirred until no tail gas is released, adjust the pH of the water layer to 6.5-7.5 with 30% sodium hydroxide solution while hot. After standing for separation, the organic layer was slowly stirred and cooled to 0-5°C, filtered, washed with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com