Ranitidine hydrochloride detection method and application thereof
A technology of ranitidine hydrochloride and its detection method, which is applied in the field of liquid chromatography, can solve the problems of severe tailing, poor peak shape of impurities, inaccurate quantification, etc., and achieve the control of improved impurity content, reduction of tailing factor, and testing high precision effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The present embodiment provides a kind of detection method of ranitidine hydrochloride, and its steps are as follows:
[0057] (1) Ranitidine tablet is mixed with mobile phase, obtains the solution to be tested;
[0058] Specifically, the ranitidine produced by a certain company is taken as the test object; it is ground into a fine powder, and an appropriate amount of fine powder (approximately equivalent to 25 mg of ranitidine) under the content determination item is accurately weighed, and placed in a 25mL volumetric flask In, add the first mobile phase to dissolve and dilute to the mark, take the filtrate as need testing solution. Accurately measure 1ml, put it in a 100ml measuring bottle, dilute to the mark with the first mobile phase, shake well, then precisely measure 5ml of the solution, put it in a 25ml measuring bottle, dilute to the mark with the first mobile phase, shake well, as Control solution.
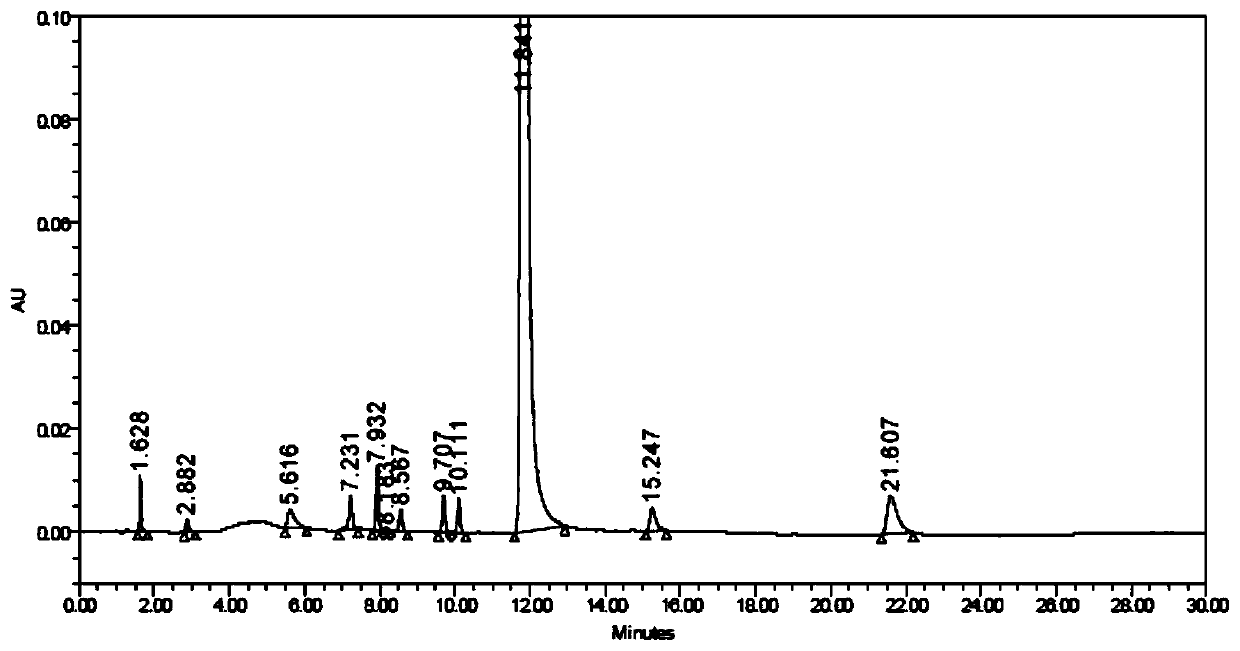
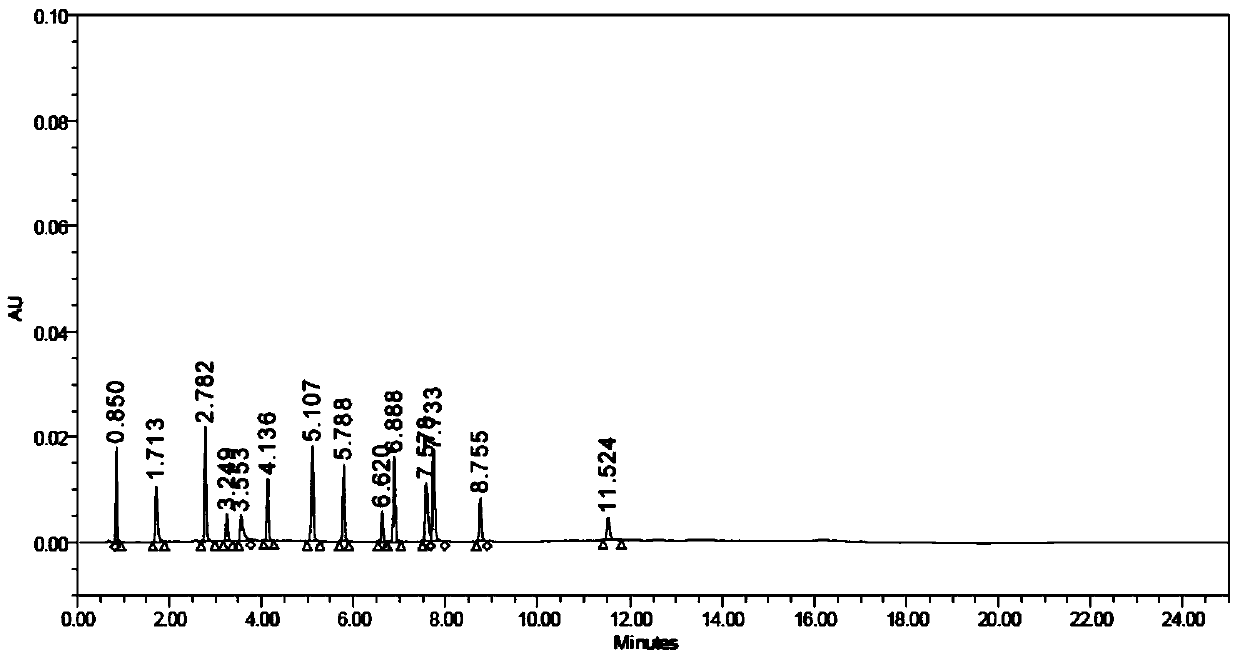
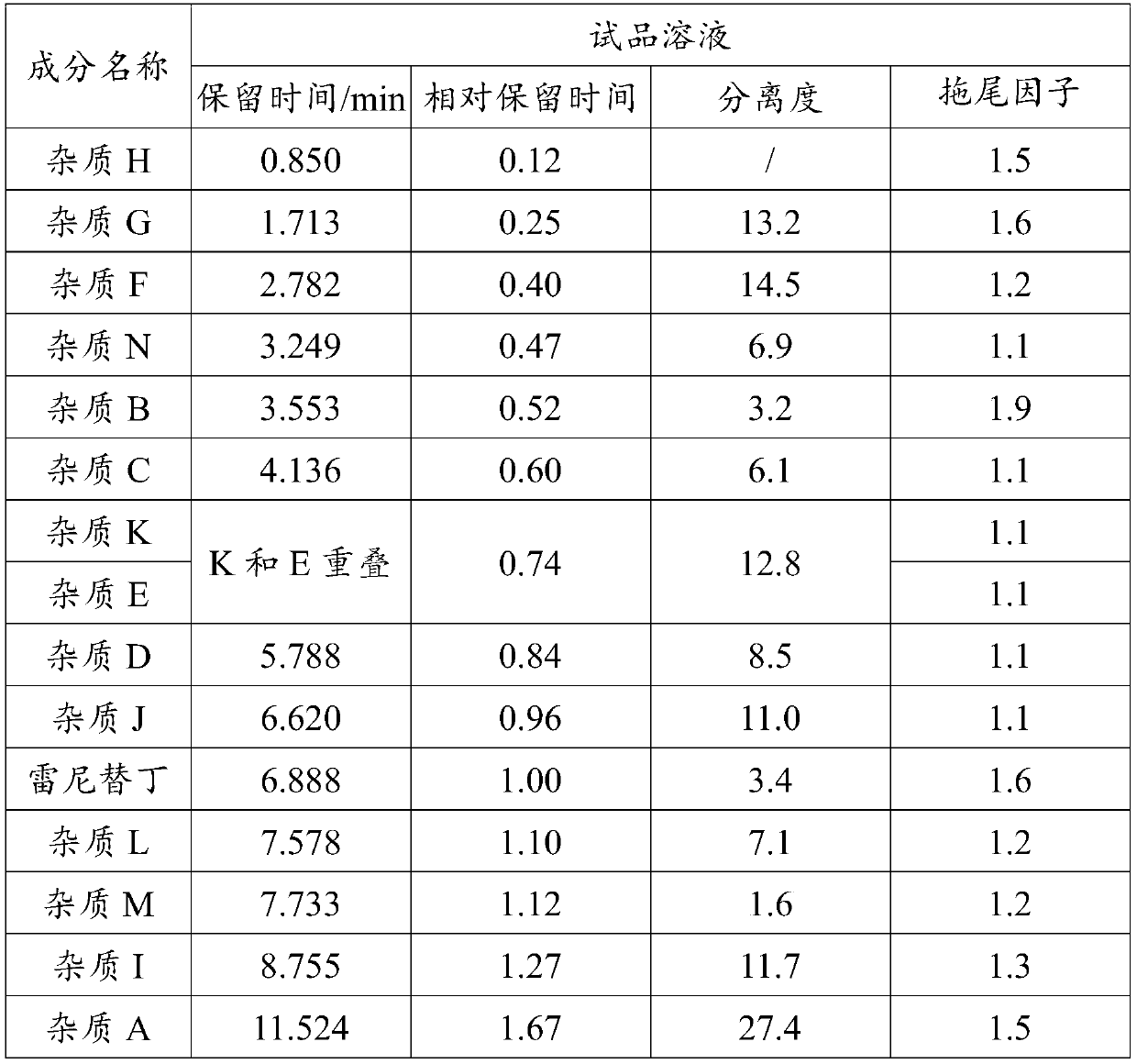
[0059] (2) The test solution is injected into a liquid chr...
Embodiment 2
[0077] Embodiment 2 quantitative limit experiment
[0078] The specific test method is as follows:
[0079] Take impurity A solution with a concentration of 500 μg / mL and other impurity solutions with a concentration of 200 μg / mL, continuously dilute with diluent and inject samples, record the chromatogram until the peak signal-to-noise ratio (S / N) ≈ 10, calculate the components Limit of quantitation and limit of quantitation concentration.
[0080] Prepare 6 quantitation limit solution injections and record the chromatograms. Calculate the peak area RSD and retention time RSD of each component. The specific experimental results are as follows:
[0081] Table 3 Quantitative Limit Determination Result Table
[0082]
[0083]
[0084] It can be seen from the above table that the quantification limit of each impurity is not more than 0.05%; the retention time RSD of each impurity in the 6 quantification limit solutions is ≤0.6%, the peak area RSD is ≤7.2%, and the signa...
Embodiment 3
[0085] Embodiment 3 linear range investigation
[0086] Take the limit of quantification solution as the LOQ level linear solution.
[0087] Take the B-N impurity solution with a concentration of 200μg / mL, accurately measure 1.5ml, 2.5ml, 4ml, 5.0ml, and 7.5ml, put them in 25ml measuring bottles, dilute with diluent to make a scale, and use them as 30%, 50% %, 80%, 100%, 150% horizontal linear solution.
[0088] Concentration preparation of impurity A by increasing: Take the mixed solution of impurity A with a concentration of 15 μg / mL and ranitidine hydrochloride with a concentration of 10 μg / mL, accurately measure 8.5ml and 12ml, put them in 25ml measuring bottles respectively, and dilute to the mark with diluent , as the linear solution after addition.
[0089] Get above-mentioned solution, adopt the method measurement of embodiment 1, record chromatogram. Perform linear regression on the concentration (X) of the above solution and the corresponding peak area (Y), and ca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Column temperature | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com