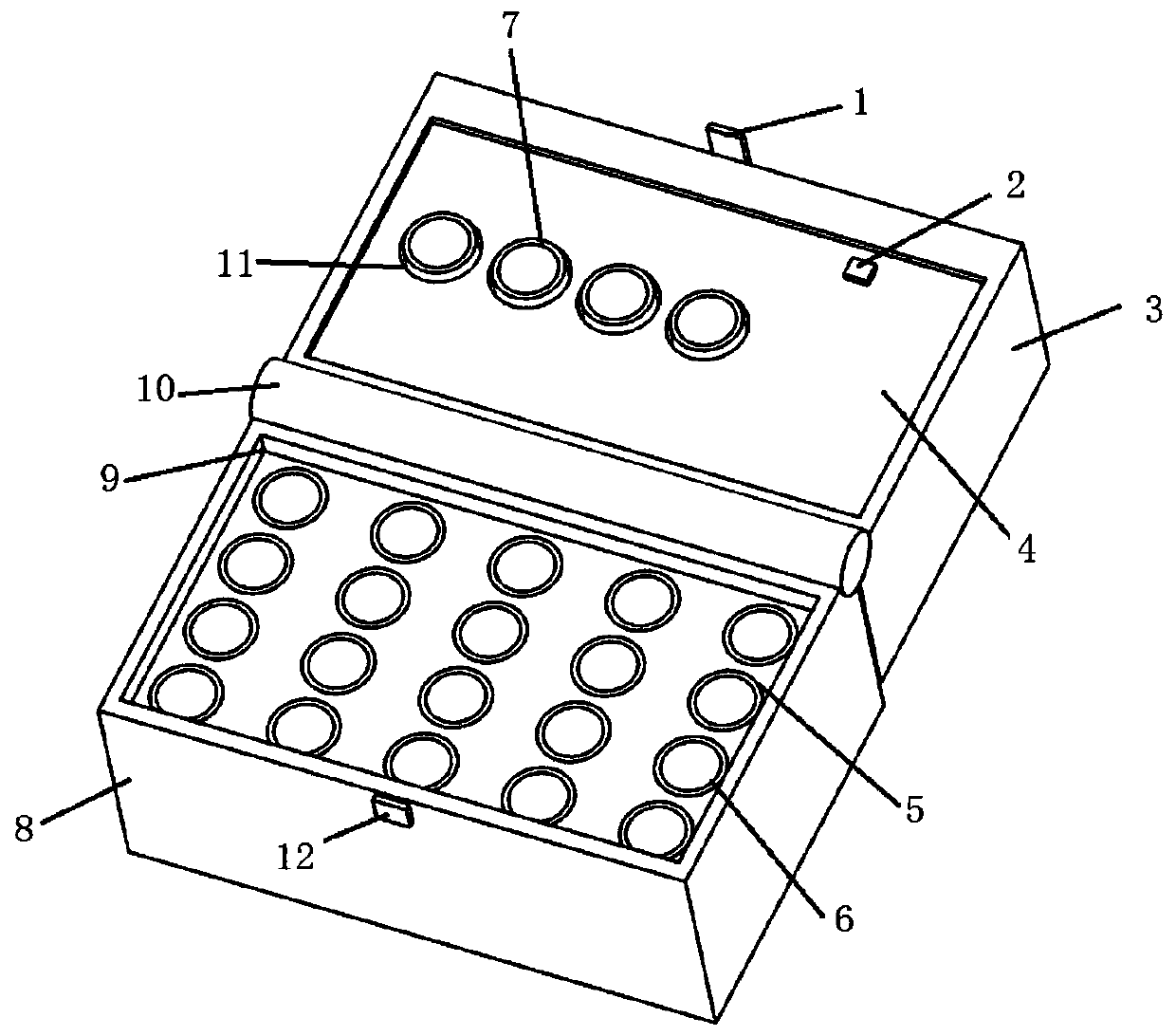
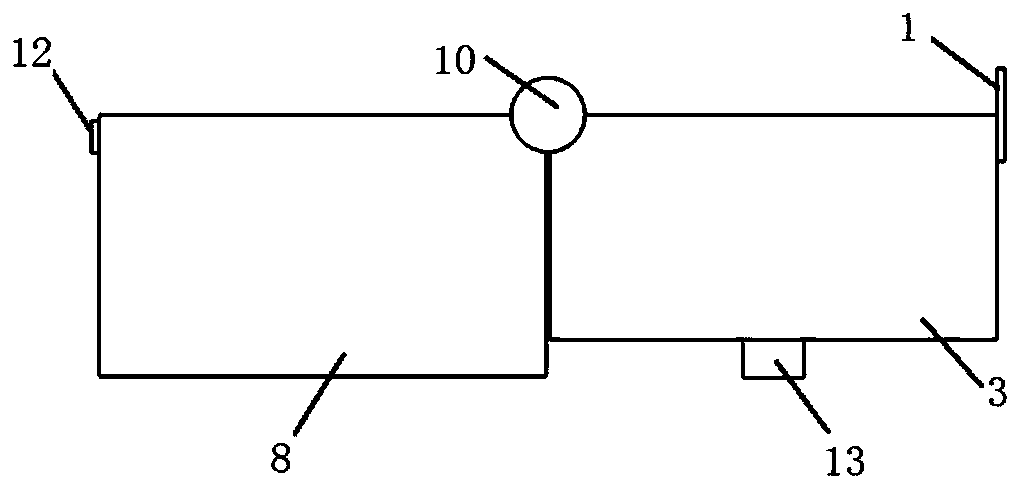
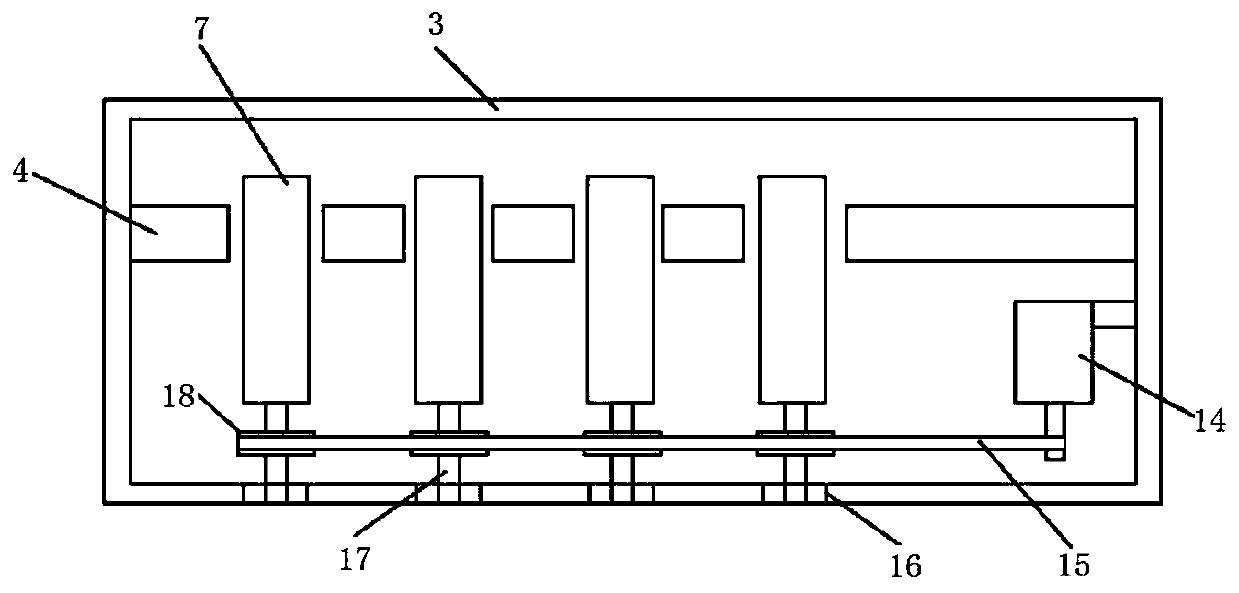
Kit for clinical examination and application
A technology of clinical testing and kits, applied in the direction of material inspection products, biological testing, test tube holders/clamps, etc., can solve the problems of large amount of reagents, small storage of kits, long reaction time, etc., to save manpower and time, The effect of saving waiting time and cost of diagnosis and treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0077] Antibody reagents are contained in a part of the first reagent tank of the present invention, and the antibody reagents are composed of sodium citrate 2.0~25g / L, citric acid 1.0~15g / L, carbamide peroxide 0.25~1.5g / L, sodium pyrophosphate 0.25~ 7.5g / L, hydrogen peroxide 1.0-1.5mL / L and polyoxyethylene lauryl ether 0.25-1.5mL / L.
[0078] Phosphoric acid diluent is filled in a part of the first reagent tank, and the phosphoric acid diluent is composed of 85% Na 2 HPO 4 , 12%NaH 2 PO 4 ·H 2 O and 3%NH 4 Cl composition, diluted with distilled water, the pH value of the phosphoric acid diluent is 7.5, and the phosphoric acid diluent needs to be passed through CO several times before use 2 , each time until the solution changes from pink to colorless, the last time before use, increase the CO of the new phosphoric acid diluent 2 Ownership.
[0079] A part of the first reagent tank is filled with Tween 80-DTPA binary compound surfactant, which is formed by mixing Tween 80 ...
Embodiment 2
[0085] The test results of all the reagents in the first reagent tank and the test results of all the reagents in the second reagent tank are obtained through the relevant information collection equipment, and then transmitted to the detector, and the test result data is compared with the database reference data of the detector. The difference points of the corresponding parameters under inspection are obtained.
[0086] Carry out corresponding judgments and exclusions on parameter differences; upload the data analysis results to the cloud data processing center; the cloud data processing center compares the case data, compares the tested data analysis results with the reference information of the cloud data processing center and generates a comparison As a result, a variant information prediction report is generated.
[0087] According to the corresponding information and the tested information, the tested test report is generated, and the high-sensitivity test result is calc...
Embodiment 3
[0089] The anti-human troponin antibody in the first reagent tank is selected from monoclonal antibodies; the troponin antibody is a mouse antibody.
[0090] The latex particles coated with anti-human troponin antibodies are prepared by mixing the antibodies with the latex latex particles and chemically reacting and cross-linking. In the preparation of the corresponding antibody-fluorescent particles, the mass ratio of the anti-antibody to the latex particles is 1:5-1:50.
[0091] The surface modification group of the latex particle of the reagent is used for chemical reaction and cross-linking with the anti-human troponin antibody, and the surface modification group of the latex particle of the reagent is a carboxyl group.
[0092] The mass-volume ratio of the latex latex particles coated with the anti-human troponin antibody has a final concentration of 0.05-0.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com