Biocompatible and degradable electrospinning asiaticoside, its preparation method and application
A technology of asiaticoside and electrospinning technology, which is applied in the field of biomaterials containing asiaticoside, can solve the problems of accelerated degradation of PLGA, influence of PLGA scaffold-mediated regeneration ability, excessive proliferation of pathological cells, etc., and achieve hydrophilicity Improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 spinning characteristic investigation
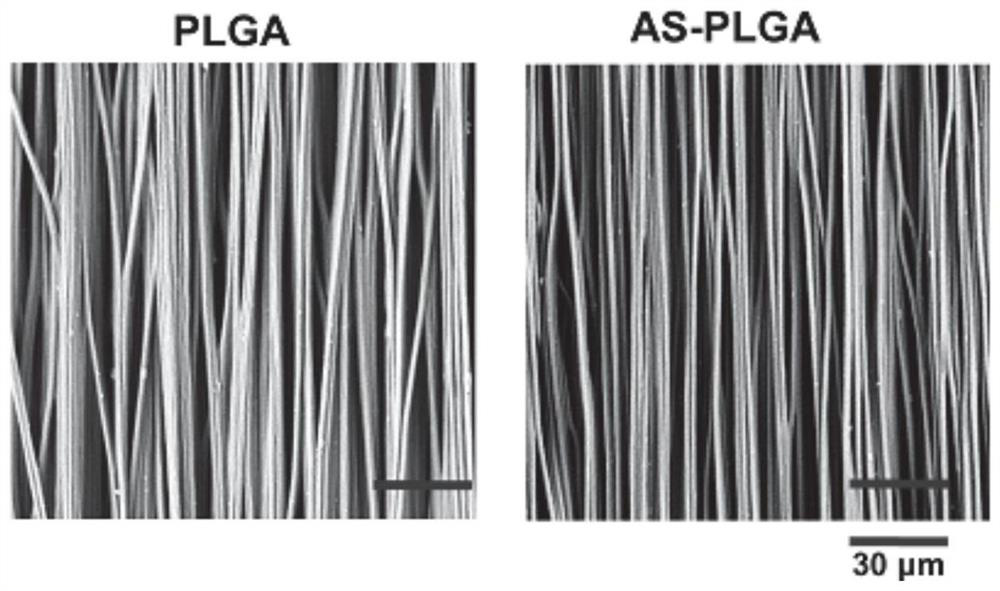
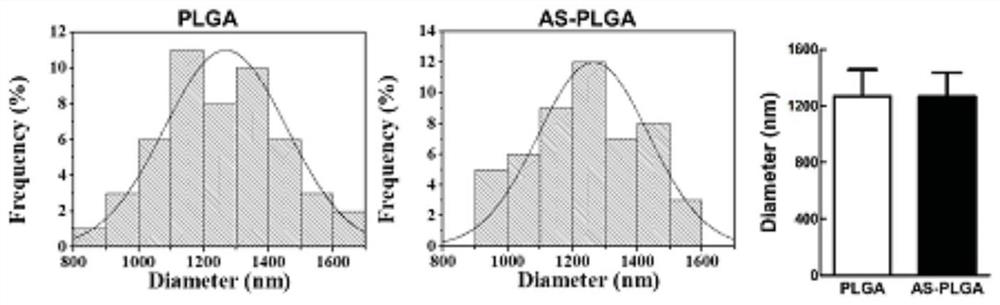
[0030] After spinning and gold-spraying, it was observed with a scanning electron microscope. Such as figure 1 Shown, the morphology of non-drug-loaded PLGA electrospinning (PLGA) and drug-loaded PLGA electrospinning (AS-PLGA). Such as figure 2 As shown, the electrospun diameter of unloaded PLGA was found to be 1265 ± 170.5 nm, and the average diameter of drug-loaded PLGA was 1269 ± 188.2 nm, which were similar. This diameter can significantly promote the proliferation and spreading of fibroblasts and the expression of collagen, which is more conducive to the repair of wounds.
[0031] Mechanical analysis of the maximum load, Young's modulus, mechanical tension and stress-strain curve of the two showed that the mechanical strength and performance of PLGA after drug loading were slightly weakened.
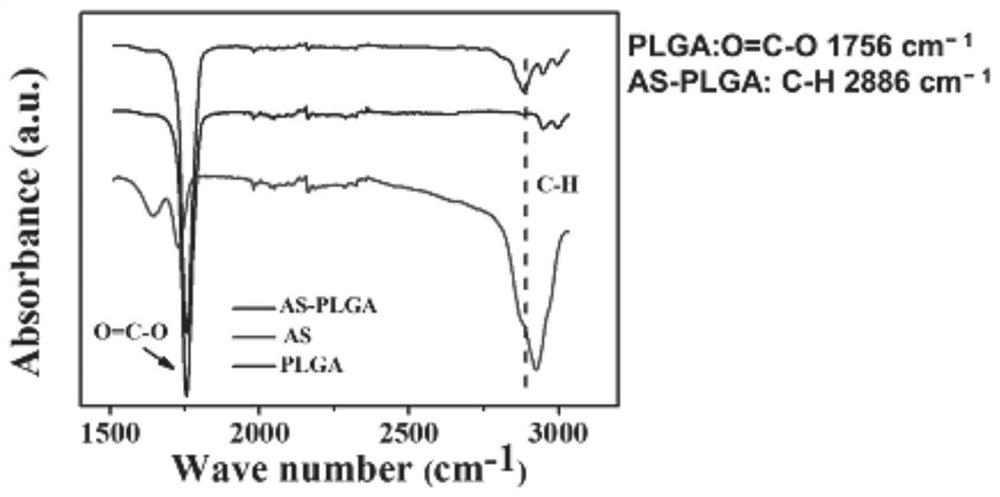
[0032] Fourier transform infrared spectroscopy (FTIR) was applied to characterize the properties of unloaded and dru...
Embodiment 2
[0034] Embodiment 2 spinning function verification
[0035] SD rats were randomly divided into two groups: non-drug-loaded PLGA electrospins and drug-loaded PLGA electrospins were implanted, respectively. SD rats were anesthetized with 10% chloral hydrate (4 ml / kg). Two PLGA electrospins were compressed into a cylindrical shape (diameter 9 mm, thickness 1 mm). Two incisions with a length of 1 cm were made on both sides of the rat spine. After the subcutaneous tissue was separated, PLGA and drug-loaded PLGA were respectively embedded, and then the wound was closed with sutures. Materials collected at 2 and 4 weeks after surgery were observed by HE staining and immunohistochemical staining. It was found that the inflammatory response produced by the host in the drug-loaded PLGA group was significantly weakened, and the material degradation was also slightly reduced. Discovered by staining for macrophage phenotypic proteins, such as Figure 5A with Figure 5B As shown, the i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com