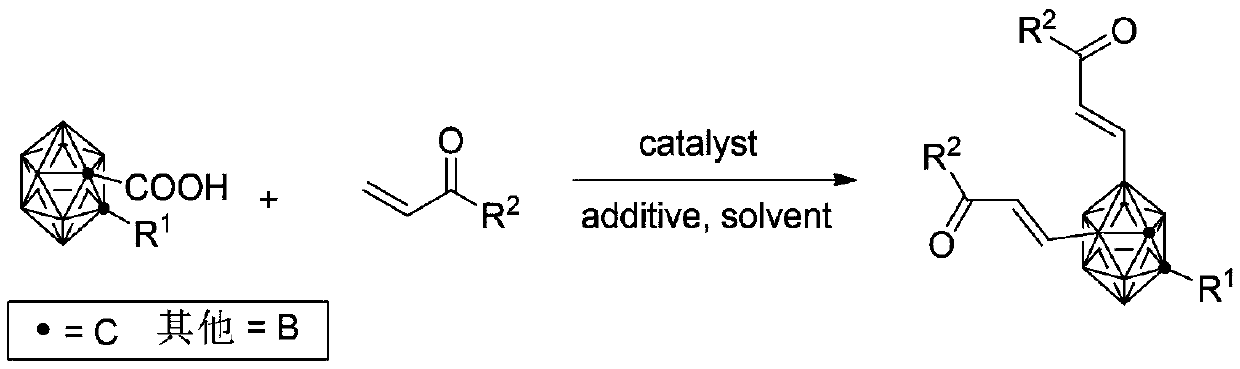
Synthesis method of B (4, 5) alkenyl substituted carborane derivative
A synthesis method, carborane technology, applied in the field of carborane synthesis, to achieve the effects of good compatibility, good universality, reaction selectivity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0027] The synthesis of embodiment 12-n-hexyl-4,5-bis(methyl acrylate)-1,2-carborane
[0028] Take a 20mL Schlenk tube and put it into a magnet, add 55mg (0.2mmol) of 1-carboxy-2-n-hexyl-o-carborane, 4mg (0.02mmol) of palladium acetate, 133mg of silver acetate (0.8mmol), and 41mg of methyl acrylate (0.48mmol), then add 5mL of 1,2-dichloroethane, and react at 70°C on an electromagnetic heating stirrer for 18 hours. After the reaction, the solvent is evaporated and purified by column chromatography to obtain 2-n-hexyl 70 mg of yl-4,5-bis(methyl acrylate)-1,2-carborane, and the yield was 88%.
[0029] Structure Identification:
[0030] 1 H NMR (500MHz, deuterated chloroform, δ / ppm) δδ6.91(d, J=17.8Hz, 2H), 6.21(d, J=17.8Hz, 2H), 3.74(s, 7H), 2.26–2.17( m,2H),1.46–1.41(m,2H),1.30–1.23(m,6H),0.88(t,J=6.9Hz,3H). 13 C NMR (126MHz, deuterated chloroform, δ / ppm) δ166.12, 133.38, 75.38, 61.15, 51.67, 38.05, 31.20, 29.16, 28.49, 22.36, 13.88. 11 B NMR (160 MHz, deuterated chloroform...
Embodiment 2~13
[0034] Examples 2-13 Synthesize 2-n-hexyl-4,5-bis(methyl acrylate)-1,2-carborane according to the same method as in Example 1, and study by adjusting parameters such as additives, solvents and time The influence of reaction conditions on product yield, experimental result sees the following table:
[0035] Example additive solvent temperature time Yield 2 Silver oxide (0.8mmol) 1,2-Dichloroethane 70℃ 18h 58% 3 Silver carbonate (0.8mmol) 1,2-Dichloroethane 70℃ 18h 73% 4 Silver trifluoroacetate (0.8mmol) 1,2-Dichloroethane 70℃ 18h 65% 5 Silver acetate (0.8mmol) 1,2-Dichloroethane 70℃ 18h 88% 6 Copper acetate (0.8mmol) 1,2-Dichloroethane 70℃ 18h 35% 7 Silver acetate (0.8mmol) N,N-Dimethylformamide 70℃ 18h <5%
Embodiment 14~27
[0037] Examples 14-27 Synthesize B(4,5) alkenyl-substituted carboranes according to the same method as in Example 2, and study the reaction conditions by adjusting the types of α, β unsaturated esters, ketones, and amides alkenylating reagents To the influence of product yield, experimental result sees the following table:
[0038] Example α, β unsaturated esters, ketones, amides alkenylating reagents Yield 14 ethyl acrylate 82 15 n-butyl acrylate 80% 16 tert-butyl acrylate 85% 17 Phenyl acrylate 77% 18 benzyl acrylate 80% 19 methyl vinyl ketone 72% 20 1-octen-3-one 65% 21 1-phenyl-2-propenyl-1-one 57% 22 1-(4-Methyl-phenyl)-2-propenyl-1-one 42% 23 1-(3-Methyl-phenyl)-2-propenyl-1-one 43% 24 1-(2-Methyl-phenyl)-2-propenyl-1-one 55% 25 1-(4-Methoxy-phenyl)-2-propenyl-1-one 63% 26 N, N-2 methacrylamide 78% 27 N-tert-butylacrylamide 82%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com