Full-spectrum high-brightness high-stability fluorescent dye as well as synthesis and application thereof
A fluorescent dye, high stability technology, applied in the field of fluorescent dyes, to achieve the effect of high cell permeability, stability improvement, and fluorescence brightness improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
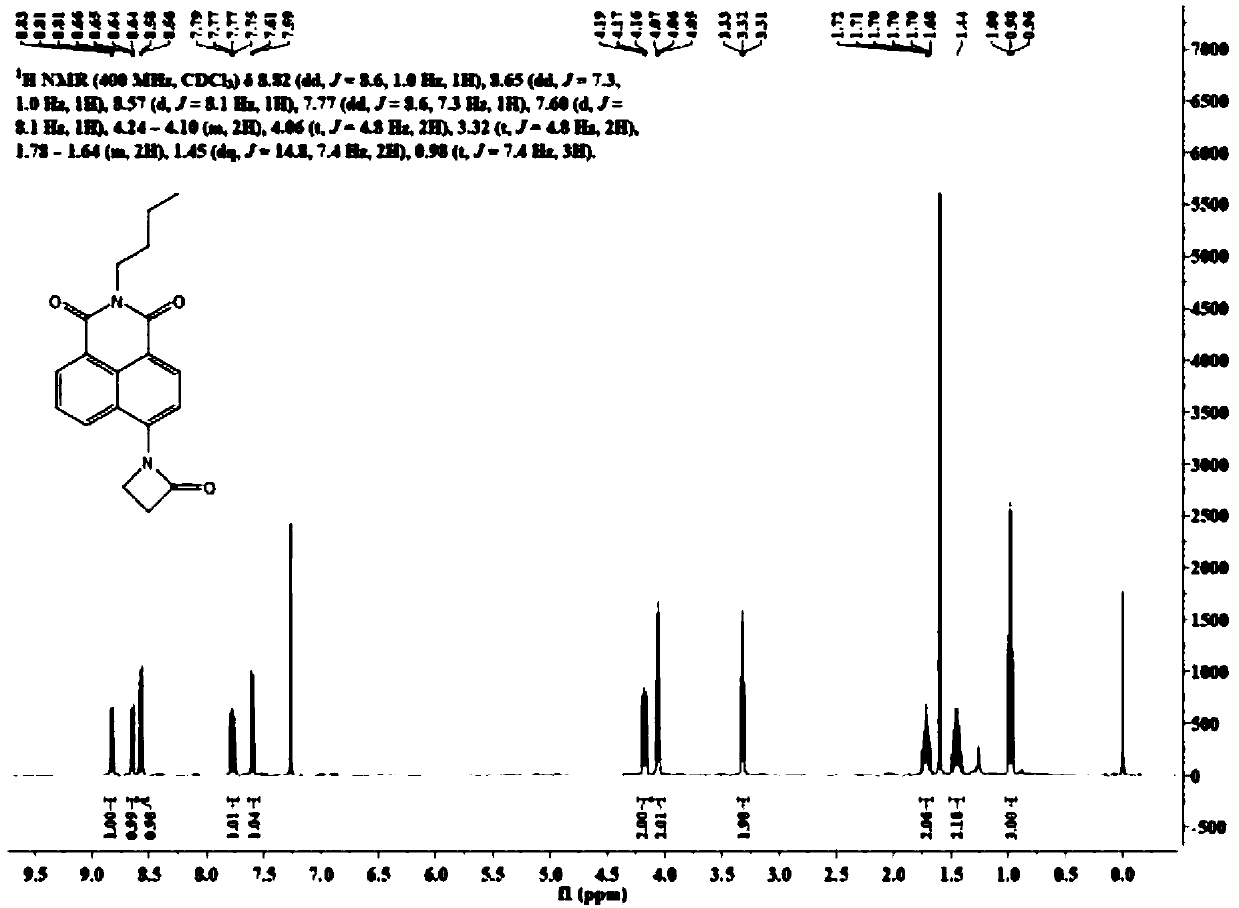
[0221] Synthesis of dye N-butyl-4-cyclobutyramido-1,8 naphthalimide (PAm)
[0222] Synthesis of intermediate N-butyl-4-(3-chloro)propionamido-1,8 naphthimide (ClPAm):
[0223]
[0224] N-butyl-4-amino-1,8-naphthimide (200 mg, 0.75 mmol) was dissolved in 100 mL of tetrahydrofuran, and 1.25 mL of 3-chloropropionyl chloride was added dropwise to the reaction solution at 0°C. After the addition is complete, the mixed solution is transferred to room temperature and reacted for 6 hours. After the solvent was removed under reduced pressure, the residue was washed with 60 mL of water and filtered with suction to obtain a white filter cake. The filter cake was washed with 25 mL of methanol and dried under vacuum to obtain N-butyl-4-(3-chloro)propionamido-1,8-naphthalene. Imide 180mg, yield 67%. The hydrogen spectrum data of the NMR spectrum are as follows:
[0225] 1 H NMR (400MHz, CD 3 CN)δ8.91(s,1H), 8.59(dd,J=7.3,0.9Hz,1H), 8.54(d,J=8.1Hz,1H), 8.52–8.48(m,1H), 8.29(d, J = 8.1 Hz, 1H), ...
Embodiment 2
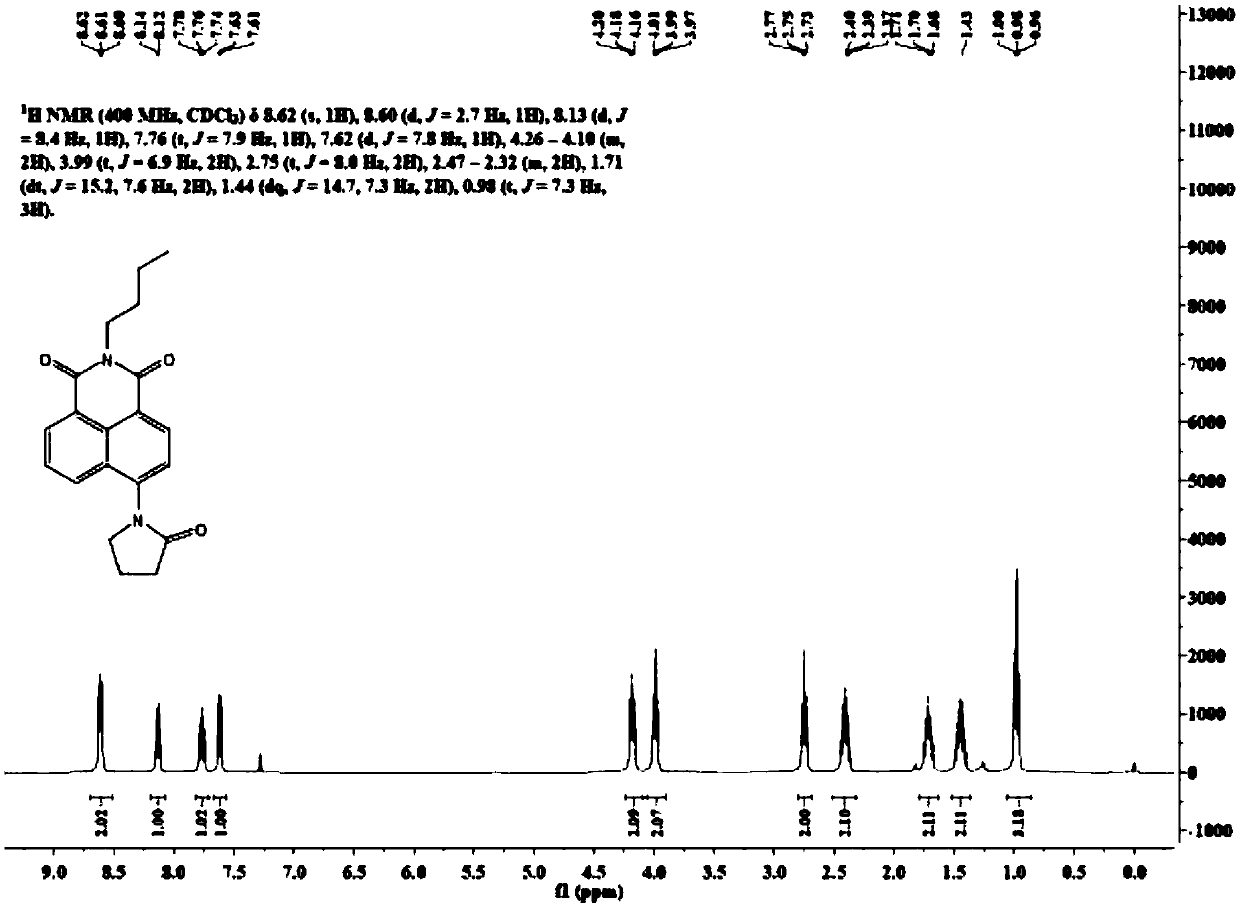
[0232] Synthesis of dye N-butyl-4-cyclopentanamido-1,8 naphthalimide (BAm)
[0233] Synthesis of intermediate N-butyl-4-(4-chloro)butyramido-1,8 naphthalimide (ClBAm):
[0234]
[0235] N-butyl-4-amino-1,8-naphthimide (400 mg, 1.49 mmol) was dissolved in 16 mL of tetrahydrofuran, and 1.6 mL of 4-chlorobutyryl chloride was added dropwise to the reaction solution at 0°C. After the addition, the mixed solution was transferred to room temperature and reacted for 10 hours. After the solvent was removed under reduced pressure, the residue was washed with 8 mL of water and filtered with suction to obtain a white filter cake. The filter cake was washed with 16 mL of methanol and dried under vacuum to obtain N-butyl-4-(4-chloro)butyramido-1,8-naphthalene. Imide 340mg, yield 61%. The hydrogen spectrum data of the NMR spectrum are as follows:
[0236] 1 H NMR(400MHz, CDCl 3 )δ8.64(d,J=7.2Hz,1H), 8.61(d,J=8.2Hz,1H), 8.42(d,J=6.8Hz,1H), 8.20(d,J=8.5Hz,1H) ,7.88(s,1H),7.84–7.75(m,1H),4.28–4.11...
Embodiment 3
[0243] Synthesis of DOAN
[0244] Synthesis of N-butyl-4-bromo-5-nitro-1,8-naphthimide (BuAN-NBr):
[0245]
[0246] 4-Bromo-5-nitro-1,8-naphthimide (1.0 g, 3.11 mmol) was dissolved in 50 mL of ethanol, and n-butylamine (250 mg, 3.43 mmol) was added dropwise. After 12 hours at 40°C, the solvent was distilled off under reduced pressure, and the residue was separated on a silica gel column (petroleum ether: dichloromethane=2:1, V / V) to obtain 620 mg of off-white solid with a yield of 53%. The hydrogen spectrum data of the NMR spectrum are as follows:
[0247] 1 H NMR(400MHz, CDCl 3 )δ8.72(d,J=7.8Hz,1H), 8.52(d,J=7.9Hz,1H), 8.21(d,J=7.9Hz,1H), 7.95(d,J=7.8Hz,1H) ,3.66(t,J=6.5Hz,2H),1.68(m,2H),1.40(m,J=7.8Hz,2H),0.94(t,J=7.9Hz,3H).
[0248] Synthesis of DOAN:
[0249]
[0250] Dissolve ethylene glycol (20mg, 0.33mmol) in 10mL dry tetrahydrofuran, add Na block (20mg, 0.89mmol) under nitrogen, add N-butyl-4-bromo-5-nitroto the reaction solution after 0.5h Base-1,8-naphthimide (120mg, 0.31...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com